Loss of a universal tRNA feature reported

tRNAs are the molecules responsible for decoding sequence information specified by messenger RNA molecules, information which is ultimately encoded by the DNA template.
tRNAHis is the specific tRNA that assists in incorporating the amino acid histidine into new proteins. Histidine residues make essential contributions to protein structure as well as the catalytic mechanisms of enzymes and must be reliably incorporated during the process of translation.
Until now, bacterial, archaeal, and eukaryotic tRNAs have always been found with an extra guanylate residue at the 5' end of the tRNA molecule. The scientists, led by Kelly Williams of VBI, have shown that tRNAs carrying the amino acid histidine in the alphaproteobacteria Sinorhizobium meliloti and Caulobacter crescentus apparently lack the universal guanylate residue.
Kelly Williams, research investigator at VBI, remarked: “The loss of a universal and apparently ancient tRNA feature in two members of the alphaproteobacteria was particularly surprising as it represents a radical departure from previously known identity rules for the histidine-carrying tRNAs.” He added: “This result implies that tRNA recognition by the enzyme adding histidine to tRNA differs considerably from similar enzymes in other organisms. We have indeed been able to detect an impact on particular regions of the histidyl-tRNA synthetase that are critical for recognizing tRNA.”
The researchers used bioinformatic tools such as a computer script – specifically written by the group – to probe the tRNA genes in the alphaproteobacteria group. Examination of the corrected tRNAHis sequences revealed that a group of alphaproteobacteria fails to encode a G (guanylate) at the -1 position of the tRNA as all other bacteria do. Amplification and tRNA sequencing approaches were used to confirm the findings.
Sinorhizobium meliloti and Caulobacter crescentus are members of the alphaproteobacteria, a group of diverse organisms whose members have successfully adopted different lifestyle and energy-yielding strategies in the course of evolution. The organisms are members of a specific group of alphaproteobacteria that comprises the Rhizobiales, Rhodobacterales, Caulobacterales, Parvularculales and Pelagibacter.
Dieter Söll, Sterling Professor of Molecular Biophysics and Biochemistry in the Department of Molecular Biophysics and Biochemistry at Yale University, commented: “The observed absence of the extra guanylate in tRNAHis is a unique case of divergence from a highly conserved ancient tRNA recognition mechanism by an aminoacyl-tRNA synthetase. It will be of special interest to examine if the loss of this tRNA feature has resulted from the presence of an RNase P enzyme that is no longer capable of catalyzing abnormal cleavage at the -1 position in the tRNAHis precursor. It will also be revealing to see how different aminoacyl-tRNA synthetases have adapted to discriminate between their cognate or related substrates and the now less distinctive tRNAHis.”
Media Contact
More Information:
http://vwww.vt.eduAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Why getting in touch with our ‘gerbil brain’ could help machines listen better
Macquarie University researchers have debunked a 75-year-old theory about how humans determine where sounds are coming from, and it could unlock the secret to creating a next generation of more…
Attosecond core-level spectroscopy reveals real-time molecular dynamics
Chemical reactions are complex mechanisms. Many different dynamical processes are involved, affecting both the electrons and the nucleus of the present atoms. Very often the strongly coupled electron and nuclear…
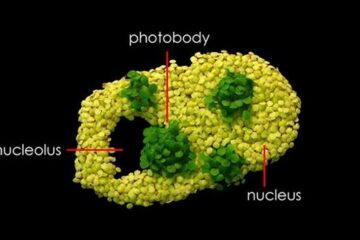
Free-forming organelles help plants adapt to climate change
Scientists uncover how plants “see” shades of light, temperature. Plants’ ability to sense light and temperature, and their ability to adapt to climate change, hinges on free-forming structures in their…