Gene-bender proteins may sway to DNA

How does the protein recognize a particular binding site” Structural changes in both the protein and DNA, sometimes with the DNA within the complex kinked or sharply bent, allow for the specific contacts needed for a tight DNA-protein fit.
Scientists think DNA is largely passive in this genetic tango. But new findings by Anjum Ansari, associate professor of biophysics at the University of Illinois at Chicago, suggest DNA may not be the wallflower that many had assumed.
To follow in real time the structural changes that accompany protein-DNA binding, Ansari and her UIC colleagues used a test protein from bacteria and applied a laser pulse lasting about 10 billionths of a second to heat up and disturb the protein-DNA complex. They watched the dynamics of the bound DNA in response to this perturbation.
Ansari's group was the first to apply the laser temperature-jump technique to study the dynamics of a protein-DNA complex.
The studies were done in collaboration with Donald Crothers, Sterling Professor Emeritus of chemistry at Yale University, who examined the protein-DNA interaction with the more traditional stopped-flow technique.
“While stopped-flow technique can capture dynamics of biomolecules occurring on millisecond time-scales or longer, the goal of this study was to extend the time-resolution down to sub-microseconds. It gave us a new time window on probing protein-DNA interactions,” Ansari said.
That broader time window, obtained in combination with the stopped-flow measurements, provided the first direct observation of DNA bending when bound to a DNA-bending protein.
“We found that the time-scales on which DNA was bending were very similar to previously reported time-scales on which individual base-pairs that hold the two DNA strands together were transiently breaking. That led us to conclude that the DNA is able to bend or kink on its own, at weak points created by the transient opening of base-pairs, and that the protein recognizes and binds tightly to the bent DNA conformation.”
Conclusions by Ansari and her colleagues deviate slightly from the conventional dogma that it is the protein that bends the DNA. She said the results raise important questions about the role that the DNA “bendability” plays in guiding the correct bending protein to the appropriate site on the DNA.
Ansari said the research adds to the basic understanding of how proteins recognize a specific binding site.
“Gaining better insights into protein-DNA interactions that control all aspects of gene regulation may prove useful for rational design of drugs to target specific sites on the DNA, whereby one can ultimately develop better gene-based therapies,” she said.
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Why getting in touch with our ‘gerbil brain’ could help machines listen better
Macquarie University researchers have debunked a 75-year-old theory about how humans determine where sounds are coming from, and it could unlock the secret to creating a next generation of more…
Attosecond core-level spectroscopy reveals real-time molecular dynamics
Chemical reactions are complex mechanisms. Many different dynamical processes are involved, affecting both the electrons and the nucleus of the present atoms. Very often the strongly coupled electron and nuclear…
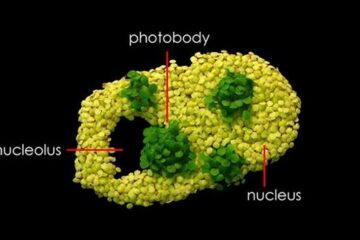
Free-forming organelles help plants adapt to climate change
Scientists uncover how plants “see” shades of light, temperature. Plants’ ability to sense light and temperature, and their ability to adapt to climate change, hinges on free-forming structures in their…