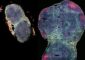
Faulty folding of protein in the environment may cause disease

Serious diseases have been shown to be related to unhealthy protein chains that occur when proteins fold ’incorrectly’. In an article in the scientific journal Proceedings of the National Academy of Science, PNAS, a research team from Uppsala University have shown that similar protein chains in our environment may hasten the process.
Under certain conditions, incorrectly folded proteins can transmit diseases from one individual to another. This is the mechanism in diseases caused by prions, such as mad cow disease or Creutzfeldt-Jakob disease. In principle prions are normal proteins, but they have an abnormal three-dimensional structure. Prions bring about infections by prompting other normal protein molecules to assume the abnormal form. These lumps then aggregate into a chain, which starts a chain reaction that ultimately causes a fatal disease.
There are other human proteins that can also change their three-dimensional structure in a similar manner and give rise to unhealthy protein chains, so-called amyloid. Amyloid contributes to the occurrence of many different diseases, such as Alzheimer‚s disease and type-2 diabetes, but it is also a serious complication of long-term inflammatory conditions such as rheumatoid arthritis.
This disease, which is called secondary or AA-amyloidosis, also occurs in mice and can be transmitted from one animal to another via a prion-like mechanism. Here, too, the infected particle is not a micro-organism but rather an incorrectly folded and chain-shaped protein, in this case AA-protein in the form of amyloid. It is still not known exactly how the incorrectly folded protein gets other normally folded protein molecules to assume the abnormal form.
Amyloid is always morbid in humans and mice, but amyloid-like chains also occur normally in our environment. Certain bacteria and fungi have amyloid-like chains on their surfaces. Silk and spider webs are other amyloid-like examples. The research team has found that such chain-shaped proteins hasten the development of AA-amyloidosis and can ’transmit’ the disease in animals under certain conditions. In other words, it seems as if the chain-like protein forms in our environment can inter-react with some of our own proteins and cause disease. Since amyloid is involved in many other diseases, the findings may indicate that environmental factors of a previously unknown type can affect and hasten the occurrence of diseases in which amyloid plays a central role.
The research was carried out by a team consisting of Dr. Katarzyna Lundmark, Karolinska Institute, Associate Professor Arne Olsén, Göteborg University, Associate Professor Gunilla T Westermark, Linköping University, and Professor Per Westermark, Uppsala University.
Media Contact
More Information:
http://www.uu.seAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles
Red light therapy for repairing spinal cord injury passes milestone
Patients with spinal cord injury (SCI) could benefit from a future treatment to repair nerve connections using red and near-infrared light. The method, invented by scientists at the University of…
Insect research is revolutionized by technology
New technologies can revolutionise insect research and environmental monitoring. By using DNA, images, sounds and flight patterns analysed by AI, it’s possible to gain new insights into the world of…

X-ray satellite XMM-newton sees ‘space clover’ in a new light
Astronomers have discovered enormous circular radio features of unknown origin around some galaxies. Now, new observations of one dubbed the Cloverleaf suggest it was created by clashing groups of galaxies….