Trojan-Horse Therapy Blocks Buildup of Alzheimer’s Plaques

A potential new therapeutic approach to Alzheimer’s disease protects brain cells in culture by drastically reducing the neurotoxic amyloid protein aggregates that are critical to the development of the disease. The treatment involves dispatching a small molecule into the cell to enlist the aid of a larger “chaperone” protein to block the accumulation of the brain-clogging protein.
The new “Trojan horse” technique overcomes a major challenge in drug design – namely, the limited ability of molecules small enough to enter a cell to interfere with interactions between much larger proteins. The researchers said it might also be possible to use this new approach to sabotage proteins central to pathogenic organisms, such as human immunodeficiency virus (HIV).
Led by Howard Hughes Medical Institute investigator Gerald R. Crabtree, the researchers reported their findings in the October 29, 2004, issue of the journal Science. First author Jason Gestwicki and senior author Isabella Graef are both members of Crabtree’s laboratory at Stanford University School of Medicine.
The plaques that clog the brains of people with Alzheimer’s disease develop through the buildup of amyloid protein chains from individual units called Aß peptide. “There have been many attempts by pharmaceutical companies to develop Aß peptide inhibitors — mainly by screening for small molecules that would bind to those aggregates and hoping that they would prevent further aggregation,” said Crabtree. “But instead, what happens in virtually all cases is that those molecules just fit right into the aggregates and don’t prevent aggregation at workable concentrations.”
The issue, he explained, applies not just to amyloid aggregation, but also to protein interactions in general. “The insurmountable problem has been that protein interactions represent the binding of two large, perfectly matched surfaces,” said Crabtree. “And small molecule drugs are only a tiny fraction of the size of those surfaces. So, even if such small molecules are constructed to bind selectively at a site between two such proteins, they either just squirt out, or the plastic surfaces of the proteins just bind around them.”
In early experiments, Roger Briesewitz, a former member of the Crabtree laboratory and HHMI fellow, who is now on the faculty at Ohio State University, had been engineering the Trojan horse approach to interfere with protein-protein interactions by designing small molecules with two binding sites. One site would bind to the protein whose interaction was to be blocked. And the other site would selectively bind to a much larger protein called a chaperone. Chaperone proteins are ubiquitous in cells and serve as “helper” molecules that guide proteins to their proper functional location in the cell.
Chaperone molecules are so plentiful in the cell that recruiting a fraction of them in such a treatment approach would not compromise their normal function, noted Crabtree.
It was Graef’s insight, said Crabtree, that the Trojan horse technique might be ideal to prevent the formation of toxic amyloid aggregates to prevent Alzheimer’s disease. “Isabella suggested that we try Aß peptide as a target because it’s small enough that a bulky chaperone protein could possibly interfere with amyloid formation from the Aß peptide,” said Crabtree.
To apply the Trojan horse approach, Gestwicki constructed a series of small “linker” molecules that would attach to a molecule called FKBP, a family of chaperone proteins found naturally at high concentrations in the cell. Gestwicki attached the other end of the linker to a molecule called Congo red, which is known to selectively bind to Aß peptide.
In test-tube studies, they found that their Trojan horse molecules did, indeed, block the growth of amyloid aggregates from their Aß peptide components. In particular, they found that the molecules inhibited growth of the shorter amyloid chains, which are believed to be more toxic to neurons. They also found that by varying the linker molecules, they could optimize the pharmaceutical properties of the Trojan horse assemblage – for example, its ability to penetrate the cell membrane to enter the cell.
In studies of the molecules’ effects on amyloid growth in cultures of neurons, the researchers confirmed that the Trojan horse molecules substantially reduced the toxicity of the amyloid by inhibiting growth of the shorter, more toxic chains of the amyloid plaque. With a second round of optimization, the scientists achieved even better results “In fact,” said Crabtree, “we achieved much better protective effects than have been achieved by pharmaceutical companies and by other academic groups using other approaches to inhibiting Aß aggregation.”
The next step will be to test the Trojan horse molecules on mouse models of Alzheimer’s disease, to determine whether the molecules have a clinical effect on progression of the disease. Crabtree said that the Trojan horse approach might complement other treatments being tested for Alzheimer’s disease. These include anti-inflammatory treatments to prevent neuronal cell death from toxic aggregates, inhibitors of aberrant molecular signaling pathways in Alzheimer’s, and vaccines to trigger antibodies to rid the brain of plaque.
Crabtree also speculated that his group’s approach could be applied widely to other clinically important protein-protein interactions, such as interfering with protein enzymes critical to replication of HIV. “HIV proteins are difficult drug targets because they can mutate rapidly to render small-molecule inhibitors inefficient,” he said. “Such drugs typically bind only to a few amino acids in the protein, which the virus can easily alter by mutation. But in our approach, we could distribute the binding over a large protein-protein interaction surface, which would be far more difficult for the virus to block by mutations affecting single amino acids.”
Media Contact
More Information:
http://www.hhmi.orgAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Why getting in touch with our ‘gerbil brain’ could help machines listen better
Macquarie University researchers have debunked a 75-year-old theory about how humans determine where sounds are coming from, and it could unlock the secret to creating a next generation of more…
Attosecond core-level spectroscopy reveals real-time molecular dynamics
Chemical reactions are complex mechanisms. Many different dynamical processes are involved, affecting both the electrons and the nucleus of the present atoms. Very often the strongly coupled electron and nuclear…
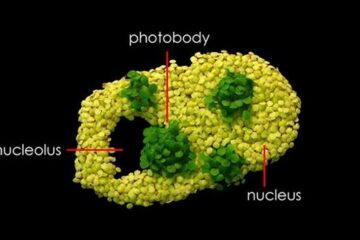
Free-forming organelles help plants adapt to climate change
Scientists uncover how plants “see” shades of light, temperature. Plants’ ability to sense light and temperature, and their ability to adapt to climate change, hinges on free-forming structures in their…