Origin of Cells for Connective Tissues of Skull & Face Challenged

Weston and co-authors from the Max Planck Institute of Immunology in Germany and the French National Scientific Research Centre at the Curie Institute document their potentially textbook-changing case in an article posted online ahead of regular publication in the Proceedings of the National Academy of Sciences.
The cells in question, they argue, do not come from a portion of embryonic neural epithelium called the neural crest, as widely believed, but rather from a distinct thin layer of epidermal epithelial cells next to it. “Our results,” Weston said, “could lead to a better understanding of the etiology of craniofacial defects, as well as the evolution of the head that distinguishes vertebrates from other creatures.”
The neural crest was first identified by classical embryologists in the late 19th and early 20th centuries and has been one of the most studied embryonic tissues. Conventional wisdom says that the neural crest gives rise to skeletal and connective tissue of the head and face, as well as a wide diversity of other stem cells that migrate to many places in the vertebrate embryo, where they spawn the cells that create the peripheral nervous system, and pigment cells in skin and hair (or scales and feathers).
The new study is part of research done over 25 years in Weston's quest to understand early development of the neural crest and explore alternative explanations for sometimes differing findings involving its assumed cell lineages. Weston noted that mutations in mice that adversely affected development of the peripheral nervous system or pigmentation did not affect craniofacial structures, whereas mutations that caused abnormal development of skeletal and connective tissue of the head and face did not alter neural crest-derived pigment or peripheral nervous system cells.
This paradox, he said, led him to wonder if different genetic programs were required to function in distinct embryonic precursors of these tissues. “In our new paper,” he said, “we finally were able to re-examine some of the underlying assumptions that have led to the conventional wisdom about the source of the embryonic cell lineages that give rise to the skeleton and connective tissue of the head and face.”
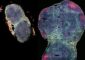
In the mouse embryo at eight days gestation, Weston and collaborators used high-resolution imaging and immunostaining techniques to identify and track the dispersal of cells known to jump start connective and skeletal tissue development. They were able to see clearly that these cells came from the non-neural layer of cells rather than from the neural crest. The same distinction also exists in chicken embryos during the first few days of gestation, Weston noted. “Looking at the right time is very important,” he said.
Weston argues that this non-neural epithelium is indeed distinct from the neural crest, because its cells contain characteristically different molecules. He and colleagues dispute suggestions that this non-neural structure is simply a sub-domain of the neural crest. “These cells emerge at a different time in development and disperse in the embryo before neural crest cells begin to migrate,” Weston said.
“New technologies let us see cell types more clearly than ever before,” said Weston, a member of the UO's Institute of Neuroscience. “We previously had discovered that a molecule that marks cell surfaces in the non-neural epithelium reveals a very sharp boundary between this non-neural epithelium and the neural tissue connected to the neural crest. In this study, we took a closer look.”
They located a population of cells in the non-neural epithelium that express other molecules that “do not appear to originate from the neural crest,” said Weston, who retired in 2001 but continued to teach in the College of Arts and Sciences until 2006. He still collaborates in some research with colleagues at the UO and at various labs around the world.
“I think our results have two important messages,” he said. “First, it is important to identify and validate — rather than ignore — assumptions; and second, because we identified an alternative embryonic cell lineage as the source of the head and facial structures, we can now more effectively analyze and understand the molecular-genetic mechanisms that regulate the normal and abnormal development of these structures.”
Co-authors with Weston were Marie Anne Breau, Thomas Pietri, and Jean Paul Thiery, all of the Curie Institute, and Marc P. Stemmler of the Max Planck Institute of Immunobiology. Pietri was a graduate student in the Curie Institute, where the project began in 2002, while Weston was on a fellowship, and now is a postdoctoral researcher with Phil Washbourne in the UO Institute of Neuroscience. Thiery, now based at the Institute of Molecular and Cell Biology of the Agency for Science, Technology and Research in Singapore, is a corresponding author with Weston on the PNAS paper.
About the University of Oregon
The University of Oregon is a world-class teaching and research institution and Oregon's flagship public university. The UO is a member of the Association of American Universities (AAU), an organization made up of 62 of the leading public and private research institutions in the United States and Canada. Membership in the AAU is by invitation only. The University of Oregon is one of only two AAU members in the Pacific Northwest.
Sources: James Weston, professor emeritus of biology, weston@uoneuro.uoregon.edu; Jean Paul Thiery, deputy director, Institute of Molecular and Cell Biology of the Agency for Science, Technology, and Research, +65 6586 9755, jpthiery@imcb.a-star.edu.sg
Links: Weston Web page: http://www.neuro.uoregon.edu/ionmain/htdocs/faculty/weston.html; UO Institute of Neuroscience: http://www.neuro.uoregon.edu/; UO College of Arts and Sciences: http://cas.uoregon.edu/
Media Contact
More Information:
http://www.uoregon.eduAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles
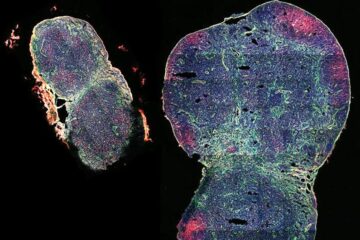
Expanding a lymph node, boosting a vaccine
A biomaterial vaccine enhances and sustains lymph node expansion following vaccination, boosting anti-tumor immunity in an animal model. Each one of us has around 600 lymph nodes (LNs) – small,…

AI to Make Crop Production More Sustainable
Drones monitoring fields for weeds and robots targeting and treating crop diseases may sound like science fiction but is actually happening already, at least on some experimental farms. Researchers from…

Cruise Ship as Data Collector
New Approaches in Ocean Observation… Scientific research – not only confined to dedicated research vessels but also from non-scientific vessels and marine infrastructures. This is one of the ideas promoted…