More Power, Longer Life, Increased Safety

A common problem with notebook computers is that the battery delivers power for far too short a time, which only gets shorter as it gets older. If you don’t take great care with your rechargeable battery at all times, its life drains away quite fast.
Improved batteries that deliver significantly more energy, last longer, and are safer are thus the subject of current research. Bruno Scrosati and Jusef Hassoun at the University of Rome have now developed a highly promising approach to a new variety of lithium-ion battery, which, as the researchers report in the journal Angewandte Chemie, may fulfill these criteria.
The dilemma is this: Mobile phones, notebook computers, smart phones, and PDAs keep getting smaller, whilst their energy requirements grow. The batteries can’t keep up. Theoretically, lithium–sulfur batteries would be the energy source of choice, because they deliver significantly more energy – by mass – than conventional lithium-ion batteries. However, their practical application suffers from the fact that their electrodes slowly dissolve, which results in a loss of capacity. Furthermore, lithium metal can form dendritic deposits that cause short circuits. This is why commercial “lithium” batteries do not contain lithium metal electrodes, but a material that can absorb and then release lithium ions, which is usually graphite. This type of lithium-ion cell supplies energy by transferring lithium ions only and delivers less energy.
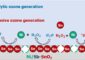
The Italian researchers would like to combine the advantages of both types of battery to make long-lived, storable, safe, and easily produced high-capacity batteries. Their new type of lithium-metal-free cell uses a cathode (negative electrode) made of a carbon/lithium sulfide composite. The organic electrolyte solution is replaced by a lithium-ion-containing liquid enclosed in a gel–polymer membrane. The polymer shields the liquid from the electrodes. The solution is also saturated with lithium sulfide. Both of these measures minimize the dissolution of the electrode components. For their anode (positive electrode), Scrosati and Hassoun selected nanoscopic tin particles that are enclosed in a protective carbon matrix.
The electrochemical process occurs as follows: At the cathode, lithium sulfide is split into elemental sulfur and lithium ions. This process releases electrons. The lithium ions migrate through the electrolyte membrane to the anode, where they take up electrons to become uncharged lithium atoms, which are then bound into an alloy by the tin nanoparticles. The process is reversible so that the battery can be charged again and again. With a specific energy of about 1100 W h/kg, the new cell surpasses all previous lithium-metal-free batteries. With this large value of energy density, this new battery may also finds its way as power source of choice for electric vehicle applications.
Author: Bruno Scrosati, Università degli Studi di Roma La Sapienza, Roma (Italy), http://www.chem.uniroma1.it/dinamico/scheda_doc1.php?userid=scrosati
Title: A High-Performance Polymer Tin Sulfur Lithium Ion Battery
Angewandte Chemie International Edition,
Permalink: http://dx.doi.org/10.1002/anie.200907324
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles
Red light therapy for repairing spinal cord injury passes milestone
Patients with spinal cord injury (SCI) could benefit from a future treatment to repair nerve connections using red and near-infrared light. The method, invented by scientists at the University of…
Insect research is revolutionized by technology
New technologies can revolutionise insect research and environmental monitoring. By using DNA, images, sounds and flight patterns analysed by AI, it’s possible to gain new insights into the world of…

X-ray satellite XMM-newton sees ‘space clover’ in a new light
Astronomers have discovered enormous circular radio features of unknown origin around some galaxies. Now, new observations of one dubbed the Cloverleaf suggest it was created by clashing groups of galaxies….