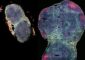
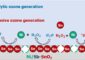
Nano-breakthrough: Solving the case of the herringbone crystal

The result gives nanotechnology researchers a new tool for controlling how objects one-millionth the size of a grain of sand arrange themselves into useful materials—and a means to discover the rest of the tool chest. A paper on the research is published online May 12 in Nature Chemistry.
“The excitement in this is not in the herringbone pattern, it's about the coupling of experiment and modeling, and how that approach lets us take on a very hard problem,” said Christopher Murray, the Richard Perry University Professor and professor of chemistry at the University of Pennsylvania.
Murray's group is renowned for making nanocrystals and arranging them into larger crystal superstructures.
Ultimately, researchers want to modify patches on nanoparticles in different ways to coax them into more complex patterns. The goal is a method that will allow people to imagine what they would like to do and then design a material with the right properties for the job.
“By engineering interactions at the nanoscale, we can begin to assemble target structures of great complexity and functionality on the macroscale,” said U-M's Sharon Glotzer, the Stuart W. Churchill Collegiate Professor of Chemical Engineering.
Glotzer introduced the concept of nanoparticle “patchiness” in 2004. Her group uses computer simulations to understand and design the patches.
Recently, Murray's team made patterns with flat nanocrystals made of heavy metals, known to chemists as lanthanides, and fluorine atoms. Lanthanides have valuable properties for solar energy and medical imaging, such as the ability to convert between high- and low-energy light.
They started by breaking down chemicals containing atoms of a lanthanide metal and fluorine in a solution, and the lanthanide and fluorine naturally began to form crystals. Also in the mix were chains of carbon and hydrogen that stuck to the sides of the crystals, stopping their growth at sizes of around 100 nanometers, or 100 millionths of a millimeter, at the largest dimensions. By using lanthanides with different atomic radii, they could control the top and bottom faces of the hexagonal crystals to be anywhere from much longer than the other four sides to non-existent, resulting in a diamond shape.
To form tiled patterns, the team spread a thin layer of nanocrystals and solvent on top of a thick fluid. As the solvent evaporated, the crystals had less space available, and they began to pack together.
The diamond shapes and the very long hexagons lined up as expected, the diamonds forming an Argyle-style grid and the hexagons matching up their longest edges like a foreshortened honeycomb. The hexagons whose sides were all nearly the same length should have formed a similar squashed honeycomb pattern, but instead, they lined up in more complicated, alternating herringbone style.
“Whenever we see something that isn't taking the simplest pattern possible, we have to ask why,” Murray said.
They posed the question to Glotzer's team.
“They've been world leaders in understanding how these shapes could work on nanometer scales, and there aren't many groups that can make the crystals we make,” Murray said. “It seemed natural to bring these strengths together.”
Glotzer and her group built a computer model that could recreate the self-assembly of the same range of shapes that Murray had produced. The simulations showed that if the equilateral hexagons interacted with one another only through their shapes, most of the crystals formed the foreshortened honeycomb pattern—not the herringbone.
“That's when we said, 'Okay, there must be something else going on. It's not just a packing problem,'” Glotzer said.
Her team, which included U-M graduate student Andres Millan and research scientist Michael Engel, then began playing with interactions between the edges of the particles. They found that that if the edges that formed the points were stickier than the other two sides, the hexagons would naturally arrange in the herringbone pattern.
The teams suspected that the source of the stickiness was those carbon and hydrogen chains—perhaps they attached to the point edges more easily. Since experimentation doesn't yet offer a way to measure the number of hydrocarbon chains on the sides of such tiny particles, Murray asked Ju Li, now the Battelle Energy Alliance Professor of Nuclear Science and Engineering at the Massachusetts Institute of Technology, to calculate how the chains would attach to the edges at a quantum mechanical level.
Li's group confirmed that because of the way that the different facets cut across the lattice of the metal and fluorine atoms, more hydrocarbon chains could stick to the four edges that led to points than the remaining two sides. As a result, the particles become patchy.
“Our study shows a way forward making very subtle changes in building block architecture and getting a very profound change in the larger self-assembled pattern,” Glotzer said. “The goal is to have knobs that you can change just a little and get a big change in structure, and this is one of the first papers that shows a way forward for how to do that.”
The paper is titled “Competition of shape and interaction patchiness for self-assembling nanoplates.”
Murray is also a professor of materials science and engineering. Glotzer is also a professor of materials science and engineering, macromolecular science and engineering, physics and applied physics. Li is also a professor of material science and engineering.
Sharon Glotzer: http://sitemaker.umich.edu/glotzergroup/home
Media Contact
More Information:
http://www.umich.eduAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles
Red light therapy for repairing spinal cord injury passes milestone
Patients with spinal cord injury (SCI) could benefit from a future treatment to repair nerve connections using red and near-infrared light. The method, invented by scientists at the University of…
Insect research is revolutionized by technology
New technologies can revolutionise insect research and environmental monitoring. By using DNA, images, sounds and flight patterns analysed by AI, it’s possible to gain new insights into the world of…

X-ray satellite XMM-newton sees ‘space clover’ in a new light
Astronomers have discovered enormous circular radio features of unknown origin around some galaxies. Now, new observations of one dubbed the Cloverleaf suggest it was created by clashing groups of galaxies….