Key step in molecular 'dance' that duplicates DNA deciphered
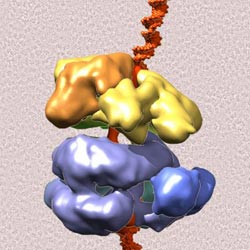
Protein machinery involved in DNA replication caught in action: The "origin recognition complex" (yellow), already activated by an initiation factor (brown), grabs onto the helicase core (purple blue) to load the helicase ring onto the DNA double helix (red). The background is a cryo-electron micrograph of many of these complexes (dark) frozen in ice.<br><br>Credit: Courtesy Brookhaven National Laboratory<br>
Building on earlier work exploring the complex choreography by which intricate cellular proteins interact with and copy DNA prior to cell division, scientists at the U.S. Department of Energy's Brookhaven National Laboratory and collaborators have captured a key step-molecular images showing how the enzyme that unwinds the DNA double helix gets drawn to and wrapped around its target. Details of the research, published in the journal Nature Structural & Molecular Biology, enhance understanding of an essential biological process and may suggest ways for stopping cell division when it goes awry.
“This was truly collaborative work where molecular biology expertise from Christian Speck's lab at Imperial College, London, Bruce Stillman's group at Cold Spring Harbor Laboratory, and the cryo-electron microscopy expertise at Brookhaven were all essential,” said Huilin Li, a biologist at Brookhaven Lab and Stony Brook University and a lead author on the paper.
“Our work is aimed at understanding the molecular details and mechanism of DNA replication at a fundamental level,” said Li, “But our findings could have important implications, possibly pointing to new ways to fight cancer, because irregularities in DNA duplication and uncontrolled cell division are hallmarks of the disease.”
The current research picks up where a study conducted last year left off [see: http://www.bnl.gov/newsroom/news.php?a=11391]. That research determined the structure of a piece of protein machinery called the “origin recognition complex” (ORC), which identifies and binds to DNA-replication “start” sites. When joined by a replication initiation factor, the ORC undergoes conformational changes that set in motion the whole replication process. The new study reveals how this previous structure recruits and interacts with the enzyme that eventually unwinds the DNA double helix into two separate strands.
“What we've uncovered in this study was a kind of missing link-what happens to this helicase enzyme before it encircles the DNA and starts unwinding the two strands,” Li said.
Speck, Group Head at the MRC Research Institute in London, commented, “Our international collaboration has now revealed how the different protein components are assembled to generate a helicase loading complex. It is fascinating to see for the first time the architecture of this molecular machine.”
Catching the molecular machinery in action is no simple task. Intermediate protein structures exist on fleeting timescales, and the interactions take place at the atomic level. Researchers working in Speck and Stillman's labs used tools of molecular biology and biochemistry to slow down the process. They purified and then remixed together pieces of the protein puzzle (including the origin recognition complex, the replication initiator, the core of the helicase, and other components) and a slow-acting energy agent so the energy-requiring reaction is unable to proceed to completion. Like dancers paused in place by a sudden stop of music, the molecular components “froze” partway through the helicase recruitment/assembly process.
Jingchuan Sun at Brookhaven then literally froze the samples, embedding them in ice, and took tens of thousands of pictures with a cryo-electron microscope. He then used computer software to reconstruct the 3-D structure from the 2-D electron microscope pictures.
“The 3-D reconstruction gave us a snapshot of the elusive intermediate structure,” Sun said.
Comparing the new structure (components of the helicase bound to the origin recognition complex) with the structures of the ORC produced last year revealed conformational changes. Binding of the helicase core components appears to shift the ORC into a spiral conformation that closely matches the spiral shape of double-stranded DNA.
“This shape-shifting of the ORC appears to be an important step in facilitating binding of the ring-shaped helicase to the DNA,” Sun said.
The scientists also note that the spiral-shaped ORC is similar to another spiral protein complex that loads a different ring structure to keep DNA polymerase enzymes from falling off the DNA while synthesizing new strands to complete the replication process.
“Both of these complexes were discovered in the Stillman lab nearly two decades ago. It's rewarding to see now that these two energy-requiring protein machines form similar spiral structures to recruit and load their 'cargo' onto DNA for these crucial steps in the replication process,” said Li.
Said co-author Stillman, president of Cold Spring Harbor Laboratory, “It is amazing how two seemingly separate steps in the process of duplicating our genome are so similar in their biochemical mechanism. Using the advanced microscope facilities at Brookhaven Lab has once again generated a surprising result.”
This research was funded by the National Institutes of Health (GM45436, GM74985), the U.K. Medical Research Council, the Japan Society for the Promotion of Science, and the Uehara Memorial Foundation. Huilin Li and the EM facility at Brookhaven Lab are partially supported by Brookhaven National Laboratory institutional funding via his joint appointment with Stony Brook University.
Related Links
Scientific paper: “Architecture of a helicase loading intermediate containing ORC-Cdc6-Cdt1-MCM2-7 on DNA reveals similarity to DNA polymerase clamp loading complexes”
Study Reveals How Protein Machinery Binds and Wraps DNA to Start Replication
One of ten national laboratories overseen and primarily funded by the Office of Science of the U.S. Department of Energy (DOE), Brookhaven National Laboratory conducts research in the physical, biomedical, and environmental sciences, as well as in energy technologies and national security. Brookhaven Lab also builds and operates major scientific facilities available to university, industry and government researchers. Brookhaven is operated and managed for DOE's Office of Science by Brookhaven Science Associates, a limited-liability company founded by the Research Foundation for the State University of New York on behalf of Stony Brook University, the largest academic user of Laboratory facilities, and Battelle, a nonprofit applied science and technology organization. Visit Brookhaven Lab's electronic newsroom for links, news archives, graphics, and more, or follow Brookhaven Lab on Twitter.
Media Contact
More Information:
http://www.bnl.govAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Sea slugs inspire highly stretchable biomedical sensor
USC Viterbi School of Engineering researcher Hangbo Zhao presents findings on highly stretchable and customizable microneedles for application in fields including neuroscience, tissue engineering, and wearable bioelectronics. The revolution in…

Twisting and binding matter waves with photons in a cavity
Precisely measuring the energy states of individual atoms has been a historical challenge for physicists due to atomic recoil. When an atom interacts with a photon, the atom “recoils” in…
Nanotubes, nanoparticles, and antibodies detect tiny amounts of fentanyl
New sensor is six orders of magnitude more sensitive than the next best thing. A research team at Pitt led by Alexander Star, a chemistry professor in the Kenneth P. Dietrich…





















