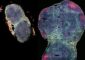
Insights into the story of the eye

Although it is well established that embryonic stem cells (ESCs) have the capacity to develop into every adult cell type in the body, mysteries abound regarding the process by which the differentiation of these cells is coordinated during the formation of complex tissues.
The eye, for example, is a very sophisticated organ, consisting of several highly organized layers of specialized cell subtypes, which initially develops from a largely homogeneous embryonic tissue known as the ectoderm. This process initiates with the formation of a bubble-like optic vesicle, which subsequently folds back on itself to form a two-layer ‘optic cup’, with the inner surface forming the sensory tissue of the neural retina and the outer surface forming the supporting tissue of the retina pigment epithelium (RPE).
How this actually happens remains unclear and controversial. “One strong hypothesis was that the invagination of the retina is caused by pushing from the lens, which itself invaginates from the surface ectoderm,” explains Yoshiki Sasai of the RIKEN Center for Developmental Biology, Kobe. He adds that although some studies support this idea, others have proven ambiguous or contradictory. However, recent stem cell research from Sasai and colleagues not only offers powerful new insights into retinal development but also provides surprising evidence in favor of an alternative hypothesis, originally proposed over 100 years ago.
Getting organized
At the turn of the 20th century, German developmental biologist Hans Spemann, a legend in the field of embryology, uncovered evidence indicating that invagination of the optic cup and subsequent lens formation occur spontaneously, without any external force. “This mechanism has been under debate for many decades since that time,” says Sasai. Using a specially designed strategy for the cultivation of mouse ESCs, his group has now provided vital support for this model by demonstrating successful differentiation of an appropriately layered and structured mouse retina in vitro, in the absence of external physical drivers.
Sasai and colleagues’ technique was an adaptation of serum-free culture of embryoid body-like aggregates (SFEBq), a cell culture system that they had developed previously to coax the development of ESCs into organized layers of cerebral cortical neurons. “The retina is another clearly stratified structure, and we tried to see whether we could achieve more complete development of layered structures that resemble postnatal tissue from stem cells,” explains Sasai.
After a week under these improved culture conditions, their ESCs developed into hollow spheres of neuroectoderm epithelial cells, precursors of nervous system tissue, which subsequently sprouted dome-shaped vesicles expressing the retina-specific gene Rax. Over the next few days, these vesicles spontaneously folded inward to form structures that closely resemble the optic cup observed in natural embryonic development (Fig. 1). Both of these newly formed layers exhibited gene expression profiles matching their natural counterparts, with no evidence for the onset of lens formation or other ectodermal influences that might represent physical triggers for this developmental process.
By closely observing this in vitro optic cup formation with a powerful fluorescence microscope, the researchers were able to characterize the details of invagination at various stages. Initially, the optical vesicles consisted exclusively of a single type of epithelial cell, but as the outermost edge of the vesicle flattened, indented and finally underwent full invagination, the cells formed distinct subpopulations that also closely resemble their counterparts in the naturally developing neural retina and RPE.
Sasai and colleagues determined that the earliest morphological changes in the vesicle appear to arise from pulling force generated by ‘motor proteins’ acting on microscopic filaments within the indenting epithelial cells. These motor proteins become less important as invagination proceeds in earnest; later stages instead appear to be powered by a pushing force generated by the physical expansion and active proliferation of cells within the developing retinal tissue.
All part of the program
To investigate the further subspecialization of neural retina layer cells, the researchers dissected their ESC-derived optic cups and cultured this cell layer independently. Within two weeks, these tissues had developed into highly stratified structures composed of a diverse array of extremely specialized cell types, each of which was spatially restricted to the appropriate level within the overall neural retina structure (Fig. 2). Aside from some minor differences, such as a relatively reduced population of color-sensing ‘cone’ photoreceptors, these in vitro-derived tissues were largely indistinguishable from a postnatal mouse retina.
In spite of Spemann’s historic predictions of retinal self-organization, Sasai admits that he and his colleagues were surprised by the extent to which such a complex organ can form in the absence of external guidance. “This means that the cells fated to become the retina have a latent, intrinsic order that allows them to form such an elaborate tissue structure by following an internal program,” he says.
Further studies will be required to determine whether these ESC-derived retinas retain full functional capacity in terms of light-sensing and signal transmission, and whether they might be used to repair retinal damage in animal models. If they pass these and other tests, such engineered tissues could prove invaluable as material for transplantation in the treatment of a variety of eye diseases.
This work may also offer useful starting points for investigating similar ‘internal programs’ that might underlie the autonomous development of stem cell populations into other complex tissues, including the elaborate and heterogeneous structures that comprise the brain. “We are now analyzing the mechanism of self-driven morphogenesis at the cellular and molecular level,” says Sasai, “and we ultimately hope to incorporate these pieces of information into a three-dimensional computer simulation.”
Reference:
Eiraku, M., Takata, N., Ishibashi, H., Kawada, M., Sakakura, E., Okuda, S., Sekiguchi, K., Adachi, T. & Sasai, Y. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 472, 51–56 (2011).
Eiraku, M., Watanabe, K., Matsuo-Takasaki, M., Kawada, M., Yonemura, S., Matsumura, M., Wataya, T., Nishiyama, A., Muguruma, K. & Sasai, Y. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell 3, 519–532 (2008).
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Why getting in touch with our ‘gerbil brain’ could help machines listen better
Macquarie University researchers have debunked a 75-year-old theory about how humans determine where sounds are coming from, and it could unlock the secret to creating a next generation of more…

Attosecond core-level spectroscopy reveals real-time molecular dynamics
Chemical reactions are complex mechanisms. Many different dynamical processes are involved, affecting both the electrons and the nucleus of the present atoms. Very often the strongly coupled electron and nuclear…
Columbia researchers “unzip” 2D materials with lasers
The new technique can modify the nanostructure of bulk and 2D crystals without a cleanroom or expensive etching equipment. In a new paper published on May 1 in the journal…