New insight into the controls on a go-to enzyme

Scientists at St. Jude Children's Research Hospital have gained new insights into regulation of one of the body's enzyme workhorses called calpains.
As the cell's molecular overachievers, calpains function in many cellular processes, including the movement of cells in tissues, the death of damaged cells, insulin secretion, and brain cell and muscle function.
The downside of this broad set of responsibilities is that defective or overactive calpains have been linked to an array of disorders, including a form of muscular dystrophy, Type 2 diabetes, gastric cancers, Alzheimer's and Parkinson's diseases, cataracts, and the death of both heart muscle in heart attacks and of brain tissue in stroke and traumatic brain injury.
“Our basic findings on calpain regulation could add useful pieces to the puzzles of these disorders and perhaps reveal targets for drugs to treat them,” said Douglas Green, Ph.D., chair of the St. Jude Department of Immunology.
Calpains are triggered by calcium flowing into the cell. This process induces the enzyme to snip apart many target proteins, as part of the cell's regulatory machinery. However, such a critical enzyme needs ultra-precise control, which is the job of another protein called calpastatin. A central question has been how calpastatin is so exquisitely specific in attaching to calpain and inhibiting it—essentially ignoring other highly similar enzymes in the cell.
In an article published in the November 20, 2008, issue of the journal Nature, Green and his colleagues report new information on the specificity of calpastatin.
“Previous studies on calpastatin had revealed how a few of the parts of the calpastatin molecule attach to calpain in the inhibition process,” said Green, the report's senior author. “However, there was no overall picture of calpastatin that revealed how it was so precise in its attachment and potent in its function.”
To obtain that overall picture, St. Jude researchers used the analytical technique of X-ray crystallography, with help from nuclear magnetic resonance (NMR) spectroscopy. In this widely used method of determining protein structure, researchers first crystallize a protein to be studied. Then, they direct X-rays through the crystal and deduce the protein structure from the diffraction pattern of those X-rays. To overcome the crystallization bottleneck, a lengthy and unpredictable variable in X-ray crystallography, the investigators used NMR spectroscopy to tailor the perfect enzyme-inhibitor complex.
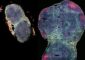
Tudor Moldoveanu, Ph.D., a postdoctoral fellow in Green's laboratory, performed X-ray structural analysis on such a protein crystal that consisted of a critical part of the calpastatin molecule attached to calpain. The structural picture obtained of the two proteins clutched together clearly revealed why calpastatin so specifically attaches to calpain.
“Calpain has multiple domains, and what we saw was that calpastatin wraps itself around pretty much every domain of calpain,” said Moldoveanu, the report's first author. This attachment not only blocks the portion of the enzyme called the active site, where calpain performs its snipping function, but also covers regions away from that site. Such a broad molecular embrace guarantees that calpastatin will potently and rapidly shut down calpain's function, Moldoveanu said. This broad embrace also guarantees that calpastatin will precisely recognize only calpains, rather than mistakenly attach to other similar enzymes in the cell.
Furthermore, the researchers discovered how calpastatin evades being chewed up by calpain. Calpastatin's survival enables it to be repeatedly recycled to inhibit calpain, making it an even more effective regulator.
The researchers' structural information also showed how calpain changes its shape once it is activated by calcium and how this transformation renders it a target of calpastatin attachment and thus inhibition.
“This new structural information on calpastatin and on calpain's conformational changes not only explains a lot about calpain's regulation,” Green said. “It also gives us information we can use to develop targets for drugs that could either activate or inhibit calpain.”
Media Contact
More Information:
http://www.stjude.orgAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles
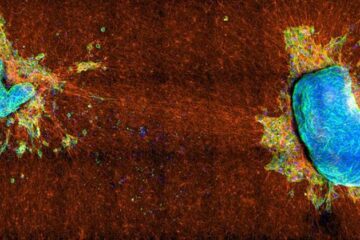
Study sheds light on cancer cell ‘tug-of-war’
How cancer cells tug against each other determines whether they can migrate elsewhere in the body. Understanding how cancerous cells spread from a primary tumor is important for any number…

Latest generation of self-dissolving stents
Magnesium implants support coronary arteries and keep them open. Constricted coronary arteries harbor dangers: Because the heart is not supplied with blood properly, this can lead to pain, cardiac arrhythmia,…

Stopping blood cancer
The MHH joint project TARGET-MPN is investigating why the disease persists and progresses in malignant bone marrow diseases from the group of myeloproliferative neoplasms despite targeted treatment. Haematopoietic stem cells…