Forgotten and lost – when proteins "shut down" our brain
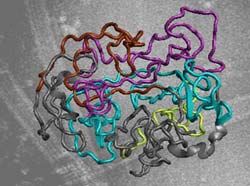
Schematic view of the tau protein structure.<br>Image: Max Planck Institute for Biophysical Chemistry / Zweckstetter<br>
Which modules of the tau protein, in neurons of Alzheimer disease patients, may act in a destructive manner were investigated by researchers from the Max Planck Institute for Biophysical Chemistry (Göttingen) and the Max Planck Unit for Structural Molecular Biology (Hamburg) with the help of Nuclear Magnetic Resonance Spectroscopy (PLoS Biology, February 17, 2009).
Coordination becomes difficult, items disappear, keeping new information in the mind is impossible. Worldwide almost 30 million people suffer from Alzheimer’s disease, a neurodegenerative, irreversible ailment which starts with memory gaps and ends in helplessness and the loss of personality. The most critical factor in developing Alzheimer’s disease is age. Most cases occur after the age of 65.
Two hallmarks are typical for Alzheimer affected brains. One of them, located between nerve cells, is amyloid plaques – extracellular protein aggregates mainly composed of a protein named beta-amyloid. The other clue is intracellular tau fibrils. In the interplay with genetic factors, the latter contribute to a disordered communication within the cell. This triggers cell death.
But the tau protein is not only harmful. Quite the contrary is the case. In its normal non-pathogenic form tau binds to microtubules, long tubular cytoskeletal building blocks, which serve as “tracks” for intracellular transport. In patients afflicted by Alzheimer’s disease or similar dementia, tau is abnormally altered. In its pathogenic form tau possesses more phosphorylated amino acids than in its normal healthy counterpart. “Our interest was focussed on how certain phosphorylated residues alter the structure of tau in a way that it can not bind to microtubules anymore” explains Markus Zweckstetter at the Max Planck Institute for Biophysical Chemistry.
Exotic among proteins
Tau is special and with most biophysical methods, such as X-ray crystallography, not analyzable. Neither heat nor acid can harm the protein. Whereas most proteins fold to adopt the structure necessary for their function, tau can do it in the absence of folded structure, is very flexible and changes its form very rapidly.
With Nuclear Magnetic Resonance Spectroscopy the scientists where able to shed light on the structural properties of tau and followed its fast motions. For the first time detailed investigations of structural changes from a large almost unfolded protein where conducted. “The financial support was granted by the DFG Research Center “Molecular Physiology of the Brain” (CMPB) in Göttingen, the Volkswagen foundation and an institute overlapping Max Planck Society project, ‘Toxic protein conformation’ “, says Christian Griesinger, head of the department of NMR-based structural biology at the Max Planck Institute.
“We can directly observe which modules of the tau protein bind to microtubules. If the protein is equipped with more phosphates than usual we can see that in this case the binding becomes significantly weaker. Tau and microtubule proteins can no longer interact” summarizes Zweckstetter. As a direct consequence the transport along the microtubule “tracks” is disturbed and nerve cell endings do not grow.
The interplay with binding partners is also possibly broken down. “We now hold the tau protein in our hands and are able to look at the interaction with its binding partners in the cell in a very detailed way”.
Tau as a drug target
Eckhard and Eva Mandelkow at the Max Planck Unit of Structural Molecular Biology in Hamburg are optimistic about using tau as a pharmaceutical target. On genetically altered mice, Eva Mandelkow and co-workers were able to show reversibility of the fatal consequences of tau aggregation. The next step for the Max Planck scientists would be the investigation of possible inhibitors which interact with the tau protein to prevent fibril formation.
Original work:
Marco D. Mukrasch, Stefan Bibow, Jegannath Korukottu, Sadasivam Jeganathan, Jacek Biernat, Christian Griesinger, Eckhard Mandelkow, Markus Zweckstetter
Structural Polymorphism of 441-residue tau at single residue resolution.
PLoS Biology, February 17, 2009.
Media Contact
More Information:
http://www.mpg.de/english/All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles
Economies take off with new airports
A global study by an SUTD researcher in collaboration with scientists from Japan explores the economic benefits of airport investment in emerging economies using nighttime satellite imagery. Be it for…
CAR T–cell immunotherapy targets
Pan-cancer analysis uncovers a new class of promising CAR T–cell immunotherapy targets. Scientists at St. Jude Children’s Research Hospital found 156 potential CAR targets across the brain and solid tumors,…

Stony coral tissue loss disease
… is shifting the ecological balance of Caribbean reefs. The outbreak of a deadly disease called stony coral tissue loss disease is destroying susceptible species of coral in the Caribbean…





















