Dodging antibiotic side effects
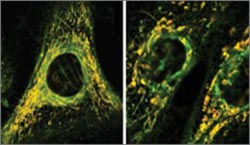
Antibiotics cause oxidative stress in cells, which leads to cellular damage. For example, in healthy cells (left), mitochondria, which are labeled yellow here, are long and highly branched. But in cells treated with the antibiotic ciprofloxacin (right), mitochondria are abnormally short and unbranched, and they do not function as well.<br><br>Credit: Sameer Kalghatgi and Catherine S. Spina<br>
A team of scientists at the Wyss Institute for Biologically Inspired Engineering at Harvard University has discovered why long-term treatment with many common antibiotics can cause harmful side effects—and they have uncovered two easy strategies that could help prevent these dangerous responses. They reported the results in the July 3rd issue of Science Translational Medicine.
“Clinical levels of antibiotics can cause oxidative stress that can lead to damage to DNA, proteins and lipids in human cells, but this effect can be alleviated by antioxidants,” said Jim Collins, Ph.D., who led the study. Collins, a pioneer of synthetic biology and Core Faculty member at the Wyss Institute, is also the William F. Warren Distinguished Professor at Boston University, where he leads the Center of Synthetic Biology.
Doctors often prescribe antibiotics freely, thinking that they harm bacteria while leaving human tissue unscathed. But over the years reports have piled up about the occasional side effects of various antibiotics, including tendonitis, inner-ear problems and hearing loss, diarrhea, impaired kidney function, and other problems.
Collins suspected these side effects occurred when antibiotics triggered oxidative stress – a condition in which cells produce chemically reactive oxygen molecules that damage the bacteria's DNA and enzymes, as well as the membrane that encloses the cell.
Collins' team had already discovered that antibiotics that kill bacteria do so by triggering oxidative stress in the bacteria. They wondered whether antibiotics caused side effects by triggering oxidative stress in the mitochondria, a bacterium-like organelle that supplies human cells with energy.
Sameer Kalghatgi, Ph.D., a former postdoctoral fellow in Collins' laboratory who is now Senior Plasma Scientist at EP Technologies in Akron, Ohio, and Catherine S. Spina, a M.D./Ph.D. candidate at Boston University and researcher at the Wyss Institute, first tested whether clinical levels of three antibiotics — ciprofloxacin, ampicillin, kanamycin –- each cause oxidative stress in cultured human cells. They found that all of these drugs were safe after six hours of treatment, but longer-term treatment of about four days caused the mitochondria to malfunction.
Kalghatgi and Spina then did a series of biochemical tests, which showed that the same three antibiotics damaged the DNA, proteins and lipids of cultured human cells — exactly what one would expect from oxidative stress.
The results mean that “doctors should only prescribe antibiotics when they're called for, and patients should only ask for antibiotics when they have a serious bacterial infection,” Collins said.
The team also treated mice with the same three antibiotics in mouse-sized doses similar to what patients receive in the clinic. Long-term treatment with each of the three antibiotics damaged the animal's lipids and caused levels of glutathione, one of the body's natural antioxidants, to fall – another sign of oxidative stress.
To make a difference in the clinic, however, the scientists still needed a way to prevent antibiotic-induced oxidative stress – or a way to remediate it as it was occurring. They found both. They were able to prevent oxidative stress by using a bacteriostatic antibiotic – an antibiotic such as tetracycline that stops bacteria from multiplying but doesn't kill them. They could also ease oxidative stress by mopping up chemically reactive oxygen molecules with an FDA-approved antioxidant called N-acetylcysteine, or NAC, that's already used to help treat children with cystic fibrosis.
The new results come on the heels of two other recent breakthroughs on antibiotic treatment from Collins' group – a report in Nature showing that viruses in the gut that infect bacteria harbor genes that confer antibiotic resistance, and another report in Science Translational Medicine showing that silver can boost the effectiveness of many widely used antibiotics.
“Jim and his team are moving at lightning speed toward unlocking the medical mysteries that stand in the way of safe and effective antibiotic treatment,” said Don Ingber, M.D., Ph.D., Wyss Institute Founding Director. “Doctors have known for years that antibiotics occasionally cause serious side effects, and Jim's new findings offer not one but two exciting new strategies that could address this long-neglected public health problem.”
Next, Collins plans more animal studies to work out the best ways to remediate oxidative stress. But since both bacteriostatic antibiotics and NAC are already FDA-approved, doctors might be using this strategy soon.
“We're interested in seeing if this could be moved toward the clinic,” Collins said.
This work was funded by the National Institutes of Health Director's Pioneer Award Program, the Howard Hughes Medical Institute, and the Wyss Institute for Biologically Inspired Engineering at Harvard University. In addition to Collins, Kalghatgi, and Spina, the research team included James C. Costello, Ph.D., a former postdoctoral fellow on Collins' team who's now an Instructor of Medicine at Harvard Medical School; Ruben Morones-Ramirez, Ph.D., a former postdoctoral fellow on Collins' team who is now a professor at Universidad Autónoma de Nuevo Leon in Mexico; Shimyn Slomovic, Ph.D., a postdoctoral fellow on Collins' team; Anthony Molina, Ph.D., Assistant Professor at Wake Forest School of Medicine; Orian Shirihai, Ph.D., Associate Professor at Boston University School of Medicine, and Marc Liesa, Ph.D., a research associate on Shirihai's team at Boston University School of Medicine.
About the Wyss Institute for Biologically Inspired Engineering at Harvard University
The Wyss Institute for Biologically Inspired Engineering at Harvard University uses Nature's design principles to develop bioinspired materials and devices that will transform medicine and create a more sustainable world. Working as an alliance among Harvard's Schools of Medicine, Engineering, and Arts & Sciences, and in partnership with Beth Israel Deaconess Medical Center, Brigham and Women's Hospital, Boston Children's Hospital, Dana Farber Cancer Institute, Massachusetts General Hospital, the University of Massachusetts Medical School, Spaulding Rehabilitation Hospital, Boston University and Tufts University, the Institute crosses disciplinary and institutional barriers to engage in high-risk research that leads to transformative technological breakthroughs. By emulating Nature's principles, Wyss researchers are developing innovative new engineering solutions for healthcare, energy, architecture, robotics, and manufacturing. These technologies are translated into commercial products and therapies through collaborations with clinical investigators, corporate alliances, and new start-ups. The Wyss Institute recently won the prestigious World Technology Network award for innovation in biotechnology.
Media Contact
More Information:
http://www.wyss.harvard.eduAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Why getting in touch with our ‘gerbil brain’ could help machines listen better
Macquarie University researchers have debunked a 75-year-old theory about how humans determine where sounds are coming from, and it could unlock the secret to creating a next generation of more…

Attosecond core-level spectroscopy reveals real-time molecular dynamics
Chemical reactions are complex mechanisms. Many different dynamical processes are involved, affecting both the electrons and the nucleus of the present atoms. Very often the strongly coupled electron and nuclear…

Free-forming organelles help plants adapt to climate change
Scientists uncover how plants “see” shades of light, temperature. Plants’ ability to sense light and temperature, and their ability to adapt to climate change, hinges on free-forming structures in their…





















