Biochemists find new treatment options for staph infections, inflammatory diseases
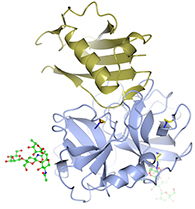
This drawing represents the crystal structure of the NSP neutrophil elastase, shown in blue, bound to the Staphylococcus aureus Eap protein, EapH1, shown in gold. Two Kansas State University biochemists have discovered that S. aureus secretes a family of proteins that prevent neutrophil serine proteases, or NSPs, from functioning — an important finding for understanding how infections are established.
Brian Geisbrecht, professor of biochemistry and molecular biophysics, and Kasra Ramyar, his research associate, are studying S. aureus, which is the cause of increasing common staph infections. Their work appears in the scientific journal Proceedings of the National Academy of Sciences of the United States of America, or PNAS, in the article “Staphylococcus aureus secretes a unique class of neutrophil serine protease inhibitors.”
While S. aureus is typically a harmless commensal organism found in the nose and skin of 30 percent of the human population, it can cause serious and deadly infections if it invades deeper tissues.
In their latest research, Geisbrecht, Ramyar and collaborators discovered that S. aureus secretes a family of proteins that prevent neutrophil serine proteases, or NSPs, from functioning — an important finding for understanding how infections are established. Neutrophils — the most abundant type of white blood cells — help prevent serious infections from occurring.
“Neutrophils are like the fire department of the immune system,” Geisbrecht said. “They are the first on the scene when a microbial infection tries to take hold.”
Neutrophils directly attack pathogens and emit biochemical signals that recruit other inflammatory immune cells to the site of infection when they release NSPs from intracellular granules.
“To our knowledge, Staph is the first example of any bacterium that secretes protease inhibitors specifically to block an aspect of the host immune response that is essential for its removal from the body,” Geisbrecht said.
The research also may lead to better treatments for inflammatory conditions, such as emphysema and chronic obstructive pulmonary disease, which are the result of dysregulated neutrophil activation in the lung.
Geisbrecht and Ramyar have closely researched neutrophils because they are the first cells to respond to infections. The biochemists' discovery that that S. aureus secretes a family of proteins — called extracellular adherence proteins, or Eaps — that block NSPs could eventually affect how staph infections are treated in the clinic.
“Understanding this interaction can not only help us design better therapies in the future, but may help make current treatment regimens work better,” Geisbrecht said.
Because these Eap proteins prevent neutrophils from working properly, they are essential for S. aureus to establish an infection.
“Our bodies respond vigorously to being invaded by S. aureus, and in order to prevent the infection from spreading, we have an arsenal of soluble molecules and white blood cells,” Ramyar said. “That is our immune system, and neutrophils are a vital part of that.”
The latest discovery puts a new twist on the past decades of research, which include studies that were done without a detailed molecular insight into how Eap proteins function. Geisbrecht, Ramyar and collaborators are now trying to better understand how their recent findings may affect interpretation of previous research.
“Bacterial pathogens like staph wouldn't be making these proteins if they weren't important to the context of infection,” Geisbrecht said. “The bacteria are trying to shut off the inflammatory response and we should be paying attention to how they're doing it.”
For this project, Kansas State University researchers collaborated with scientists from University Medical Center Utrecht in the Netherlands; Saarland University Medical Center in Germany; University of Veterinary Medicine in Hannover, Germany; and the University of Missouri, Kansas City.
The Kansas State University researchers provided experimental tools, biochemical information and the atomic resolution crystal structure of a complex formed between an S. aureus Eap protein and a human NSP. They received support from the National Institutes of Health.
Sources
Brian Geisbrecht
785-532-3154
geisbrechtb@k-state.edu
Kasra Ramyar
785-532-3154
kramyar@k-state.edu
Media Contact
More Information:
http://www.k-state.edu/media/newsreleases/sept14/pnas9214.htmlAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Microscopic basis of a new form of quantum magnetism
Not all magnets are the same. When we think of magnetism, we often think of magnets that stick to a refrigerator’s door. For these types of magnets, the electronic interactions…

An epigenome editing toolkit to dissect the mechanisms of gene regulation
A study from the Hackett group at EMBL Rome led to the development of a powerful epigenetic editing technology, which unlocks the ability to precisely program chromatin modifications. Understanding how…

NASA selects UF mission to better track the Earth’s water and ice
NASA has selected a team of University of Florida aerospace engineers to pursue a groundbreaking $12 million mission aimed at improving the way we track changes in Earth’s structures, such…





















