Antibacterial protein’s molecular workings revealed

Vanderbilt investigators now report new insights to the workings of calprotectin — including a detailed structural view of how it binds the metal manganese. Their findings, published online before print in the Proceedings of the National Academy of Sciences, could guide efforts to develop novel antibacterials that limit a microbe’s access to metals.
The increasing resistance of bacteria to existing antibiotics poses a severe threat to public health, and new therapeutic strategies to fight these pathogens are needed.
The idea of “starving” bacteria of metal nutrients is appealing, said Eric Skaar, Ph.D., MPH, associate professor of Pathology, Microbiology and Immunology.
In a series of previous studies, Skaar, Walter Chazin, Ph.D., and Richard Caprioli, Ph.D., demonstrated that calprotectin is highly expressed by host immune cells at sites of infection. They showed that calprotectin inhibits bacterial growth by “mopping up” the manganese and zinc that bacteria need for replication.
Now, the researchers have identified the structural features of calprotectin’s two metal binding sites and demonstrated that manganese binding is key to its antibacterial action.
Calprotectin is a member of the family of S100 calcium-binding proteins, which Chazin, professor of Biochemistry and Chemistry, has studied for many years. Chazin and postdoctoral fellow Steven Damo, Ph.D., used existing structural data from other S100 family members to zero in on calprotectin’s two metal binding sites. Then, they selectively mutated one site or the other.
They discovered that calprotectin with mutations in one of the two sites still bound both zinc and manganese, but calprotectin with mutations in the other site only bound zinc.
The researchers recognized that these modified calprotectins — especially the one that could no longer bind manganese — would be useful tools for determining the importance of manganese binding to calprotectin’s functions, Chazin noted.
Thomas Kehl-Fie, Ph.D., a postdoctoral fellow in Skaar’s group, used these altered calprotectins to demonstrate that the protein’s ability to bind manganese is required for full inhibition of Staphylococcus aureus growth. The investigators also showed that Staph bacteria require manganese for a certain process the bacteria use to protect themselves from reactive oxygen species.
“These altered calprotectin proteins were key to being able to tease apart the importance of the individual metals — zinc and manganese – to the bacterium as a whole and to metal-dependent processes within the bacteria,” Skaar said. “They’re really powerful tools.”
Skaar explained that calprotectin likely binds two different metals to increase the range of bacteria that it inhibits. The investigators tested the modified calprotectins against a panel of medically important bacterial pathogens.
“Bacteria have different metal needs,” Skaar said. “Some bacteria are more sensitive to the zinc-binding properties of calprotectin, and others are more sensitive to the manganese-binding properties.”
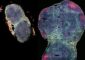
To fully understand how calprotectin binds manganese, Damo and Chazin — with assistance from Günter Fritz, Ph.D., at the University of Freiburg in Germany — produced calprotectin crystals with manganese bound and determined the protein structure. They found that manganese slips into a position where it interacts with six histidine amino acids of calprotectin.
It’s really beautiful; no one’s ever seen a protein chelate (bind) manganese like this,” Chazin said.“It’s really beautiful; no one’s ever seen a protein chelate (bind) manganese like this,” Chazin said.
The structure explains why calprotectin is the only S100 family member that binds manganese and has the strongest antimicrobial action, and it may allow researchers to design a calprotectin that only binds manganese (not zinc). Such a tool would be useful for studying why bacteria require manganese — and then targeting those microbial processes in new therapeutic strategies, Chazin and Skaar noted.
“We do not know all of the processes within Staph that require manganese; we just know if they don’t have it, they die,” Skaar said. “If we can discover the proteins in Staph that require manganese — the things that are required for growth — then we can target those proteins.”
The team recently was awarded a five-year, $2 million grant from the National Institute of Allergy and Infectious Diseases (AI101171) to advance their studies of calprotectin and how it works to limit bacterial infections and in other inflammatory conditions.
“Nature stumbled onto an interesting antimicrobial strategy,” Chazin said. “Our goal is to really tease apart the importance of metal binding to all of calprotectin’s different roles — and to take advantage of our findings to design new antibacterial agents.”
The research was supported by grants from the National Institutes of Health (CA009582, HL094296, AI091771, AI069233, AI073843, GM062122). Skaar holds the Ernest W. Goodpasture Chair in Pathology; Chazin holds the Chancellor’s Chair in Biochemistry and Chemistry and is director of the Vanderbilt Center for Structural Biology.
Contact:
Leigh MacMillan, (615) 322-4747
leigh.macmillan@vanderbilt.edu
Media Contact
More Information:
http://www.vanderbilt.eduAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles
Red light therapy for repairing spinal cord injury passes milestone
Patients with spinal cord injury (SCI) could benefit from a future treatment to repair nerve connections using red and near-infrared light. The method, invented by scientists at the University of…
Insect research is revolutionized by technology
New technologies can revolutionise insect research and environmental monitoring. By using DNA, images, sounds and flight patterns analysed by AI, it’s possible to gain new insights into the world of…

X-ray satellite XMM-newton sees ‘space clover’ in a new light
Astronomers have discovered enormous circular radio features of unknown origin around some galaxies. Now, new observations of one dubbed the Cloverleaf suggest it was created by clashing groups of galaxies….