How Alzheimer's could occur
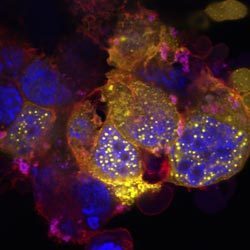
The fluorescence microscopy image shows several cells grown in the laboratory. In the nucleus (here colored blue), the protein FE65 has fused with other proteins such as BLM to form spherical structures that are seen in yellow. The cytoskeleton of the cell, a very flexible mesh in the cytoplasm, which is formed out of proteins, is shown in red.<br><br>Credit: © Thorsten Müller
A new hypothesis has been developed by researchers in Bochum on how Alzheimer's disease could occur. They analysed the interaction of the proteins FE65 and BLM that regulate cell division. In the cell culture model, they discovered spherical structures in the nucleus that contained FE65 and BLM.
The interaction of the proteins triggered a wrong signal for cell division. This may explain the degeneration and death of nerve cells in Alzheimer's patients. The team led by Dr. Thorsten Müller and Prof. Dr. Katrin Marcus from the Department of Functional Proteomics in cooperation with the RUB's Medical Proteome Centre headed by Prof. Helmut E. Meyer reported on the results in the “Journal of Cell Science”.
Components of spherical structures in the nucleus identified
The so-called amyloid precursor protein APP is central to Alzheimer's disease. It spans the cell membrane, and its cleavage products are linked to protein deposits that form in Alzheimer patients outside the nerve cells. APP anchors the protein FE65 to the membrane, which was the focus of the current study. FE65 can migrate into the nucleus, where it plays a role in DNA replication and repair. Based on cells grown in the laboratory, the team led by Dr. Müller established that FE65 can unite with other proteins in the cell nucleus to form spherical structures, so-called “nuclear spheres”. Video microscopy showed that these ring-like structures merge with each other and can thus grow. “By using a special cell culture model, we were able to identify additional components of these spheres”, says Andreas Schrötter, PhD student in the working group Morbus Alzheimer at the Institute for Functional Proteomics. Among other things, the scientists found the protein BLM, which is known from Bloom's syndrome – an extremely rare hereditary disease, which is associated with dwarfism, immunodeficiency, and an increased risk of cancer. BLM is involved in DNA replication and repair in the nucleus.
The amount of FE65 determines the amount of BLM in the cell nucleus
Müller's team took a closer look at the function of FE65. By means of genetic manipulation, the researchers generated cell cultures, in which the FE65-production was reduced. A smaller amount of FE65 thus generated a smaller amount of the protein BLM in the nucleus. Instead, BLM collected in another area of the cell, the endoplasmic reticulum. In addition, the researchers found a lower rate of DNA replication in the genetically modified cells. In this way, FE65 influences the replication of the genetic material via the BLM protein. When the researchers cranked up the FE65-production again, the amount of BLM in the nucleus also increased again.
FE65 as a possible trigger for Alzheimer's
In patients with Alzheimer's disease, the protein APP, an interaction partner of FE65, changes. The interaction of the two molecules is important for the transport of FE65 into the nucleus, where it regulates cell division in combination with BLM. Müller's team assumes that the altered APP-FE65 interaction mistakenly sends the cells the signal to divide. Since nerve cells normally cannot divide, they degenerate instead and die. “This hypothesis, which we pursue in the working group Morbus Alzheimer, also delivers new starting points for potential therapies, which are urgently needed for Alzheimer's disease,” says Dr. Mueller. In the future, the team will also investigate whether and how the amount of BLM is altered in Alzheimer's patients compared to healthy subjects.
Bibliographic record
A. Schroetter, T. Mastalski, F.M. Nensa, M. Neumann, C. Loosse, K. Pfeiffer, F. El Magraoui, H.W. Platta, R. Erdmann, C. Theiss, J. Uszkoreit, M. Eisenacher, H.E. Meyer, K. Marcus, T. Mueller (2013): FE65 regulates and interacts with the Bloom syndrome protein in dynamic nuclear spheres – potential relevance to Alzheimer's disease, Journal of Cell Science, doi 10.1242/jcs.121004
Further information
Dr. Thorsten Müller
Functional Proteomics
Medical Proteome Center at the Ruhr-Universität
44780 Bochum, Germany
Tel. 0234/32-29265
E-mail: thorsten.t.mueller@rub.de
Editor: Palina Turok
Click for more:
Functional Proteomics, Working Group Morbus Alzheimer http://funktionelle-proteomik.de/de/arbeitsgruppen/morbusalzheimer.html
Medical Proteome Center http://www.medizinisches-proteom-center.de
Media Contact
More Information:
http://www.ruhr-uni-bochum.deAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Microscopic basis of a new form of quantum magnetism
Not all magnets are the same. When we think of magnetism, we often think of magnets that stick to a refrigerator’s door. For these types of magnets, the electronic interactions…

An epigenome editing toolkit to dissect the mechanisms of gene regulation
A study from the Hackett group at EMBL Rome led to the development of a powerful epigenetic editing technology, which unlocks the ability to precisely program chromatin modifications. Understanding how…

NASA selects UF mission to better track the Earth’s water and ice
NASA has selected a team of University of Florida aerospace engineers to pursue a groundbreaking $12 million mission aimed at improving the way we track changes in Earth’s structures, such…





















