Technique simplifies the creation of high-tech crystals
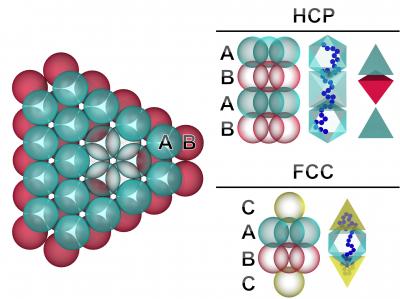
In this graphic representation of the analysis, colloids form the initial two layers of a crystal, at left. With the addition of the third layer, the crystal forms one of two possible shapes, which are shown at right. The vertical blue line to the right of each crystal shows a representative shape of the polymer when confined in the voids between colloids in each crystal. Credit: Nathan Mahynski et al
Now, researchers at Princeton and Columbia universities have proposed a new method that could allow scientists to customize and grow these specialized materials, known as photonic crystals, with relative ease.
“Our results point to a previously unexplored path for making defect-free crystals using inexpensive ingredients,” said Athanassios Panagiotopoulos, the Susan Dod Brown Professor of Chemical and Biological Engineering and one of the paper's authors. “Current methods for making such systems rely on using difficult-to-synthesize particles with narrowly tailored directional interactions.”
In an article published online July 21 in the journal Nature Communications, the researchers proposed that photonic crystals could be created from a mixture in which particles of one type are dispersed throughout another material. Called colloidal suspensions, these mixtures include things like milk or fog. Under certain conditions, these dispersed particles can combine into crystals.
Creating solids from colloidal suspensions is not a new idea. In fact, humans have been doing it since the invention of cheese and the butter churn. But there is a big difference between making a wheel of cheddar and a crystal pure enough to split light for an optical circuit.
One of the main challenges for creating these optical crystals is finding a way to create uniform shapes from a given colloidal mixture. By definition, crystals' internal structures are arranged in an ordered pattern. The geometry of these patterns determines how a crystal will affect light. Unfortunately for optical engineers, a typical colloidal mixture will produce crystals with different internal structures.
In their paper, the researchers demonstrate a method for using a colloidal suspension to create crystals with the uniform structures needed for high-end technologies.
Essentially, the researchers showed that adding precisely sized chains of molecules – called polymers – to the colloid mixture allows them to impose order on the crystal as it forms.
“The polymers control what structures are allowed to form,” said Nathan Mahynski, a graduate student in chemical and biological engineering at Princeton and the paper's lead author. “If you understand how the polymer interacts with the colloids in the mixture, you can use that to create a desired crystal.”
The researchers created a computer model that simulated the formation of crystals based on principles of thermodynamics, which state that any system will settle into whatever structure requires the least energy. Panagiotopoulos's group analyzed the equilibrium state of different possible crystal shapes to understand how they were affected by the presence of different polymers.
They found that when the crystals formed, tiny amounts of polymer were trapped between the colloids as they came together. It looks like mortar in a stone wall, although the researchers say the polymer has no adhesive property. These polymer-filled spaces, called interstices, play a key role in determining the energy state of a crystal.
“Changing the polymer affects which crystal form is most stable,” Mahynski said. “As the crystal forms, the polymer helps set the crystal's shape.”
The polymer and the forming crystal work like a lock and key – they fit together in the crystal structure with the lowest energy state. Because of this, scientists can use their knowledge of polymer physics to tailor crystal structures.
Because the researchers conducted their analysis using computer modeling, they cautioned that experimentally reproducing the results in a lab presents some difficult challenges. One early concern involved the uniformity of colloids in the suspension; models often assume colloids are all the same shape and size but this rarely occurs in natural systems. Anticipating this, the researchers tested their theory using a non-uniform solution.
“One of the things we did in our study was to look at realistic systems, that were realizable in the lab, and it appears that the phenomenon that we describe is robust,” said Sanat Kumar, a professor and chair of chemical engineering at Columbia and a researcher on the project. “That tells us that there is no conceptual problem to realizing this in the lab.”
Gravity could also pose a problem for experimenters, although Kumar pointed out that similar experiments have overcome this difficulty. Gravity causes crystals to filter to the bottom of a container and pack in mismatched layers. This can make it very difficult, and perhaps impractical, to try to produce the crystals by using a colloid mix in a tank.
But Mahynski said there are several techniques that could avoid problems. For example, to deal with gravity, researchers could create the crystals in a very thin film. That approach should avoid the disruption of gravity pulling the crystals to the bottom.
It also could be important to select the right size of colloid and type of polymer for a successful experimental result. Panagiotopoulos said that one possible path for these ideas to be experimentally verified will be to use polymer chains that are stiff, such as double-stranded DNA, together with micrometer-sized colloids.
Besides Panagiotopoulos, Kumar and Mahynski, the paper's authors also included Dong Meng, a postdoctoral researcher at Columbia. Support for the project was provided in part by the National Science Foundation.
Media Contact
More Information:
http://www.princeton.edu/main/All latest news from the category: Materials Sciences
Materials management deals with the research, development, manufacturing and processing of raw and industrial materials. Key aspects here are biological and medical issues, which play an increasingly important role in this field.
innovations-report offers in-depth articles related to the development and application of materials and the structure and properties of new materials.
Newest articles
Red light therapy for repairing spinal cord injury passes milestone
Patients with spinal cord injury (SCI) could benefit from a future treatment to repair nerve connections using red and near-infrared light. The method, invented by scientists at the University of…
Insect research is revolutionized by technology
New technologies can revolutionise insect research and environmental monitoring. By using DNA, images, sounds and flight patterns analysed by AI, it’s possible to gain new insights into the world of…

X-ray satellite XMM-newton sees ‘space clover’ in a new light
Astronomers have discovered enormous circular radio features of unknown origin around some galaxies. Now, new observations of one dubbed the Cloverleaf suggest it was created by clashing groups of galaxies….