Researchers find new mechanism governing particle growth in nanocomposites
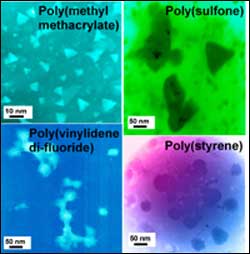
Images show how the size and shape of iron oxide nanoclusters are controlled by interfacial interactions with polymers having various functional groups.
Controlling nanoparticle size
Because the properties of nanoparticles depend so closely on their size, size distribution and morphology, techniques for controlling the growth of these tiny structures is of great interest to materials researchers today.
A research team from the Georgia Institute of Technology and Drexel University has discovered a surprising new mechanism by which polymer materials used in nanocomposites control the growth of particles. Reported on August 28th at the 230th national meeting of the American Chemical Society, the findings could provide a new tool for controlling the formation of nanoparticles.
Growing particles within the confinement of polymer-based structures is one technique commonly used for controlling nanoparticle growth. After formation of the particles, the polymer matrix can be removed – or the resulting nanocomposite used for a variety of applications.
In a series of experiments, the research team found a strong relationship between the chemical reactivity of the polymer and the size and shape of resulting nanoparticles.
“We have concentrated on the reactivity of the polymeric matrix and how that influences the growth of particles,” explained Rina Tannenbaum, an associate professor in Georgia Tech’s School of Materials Science and Engineering. “We found that in the melt the key parameter influencing particle size is actually the type of interaction with the polymer. The molecular weight of the polymer and the synthesis temperature are almost insignificant.”
In a series of experiments, Tannenbaum and her collaborators created iron oxide nanoparticles within polymer films of different types, including polystyrene, poly(methyl methacrylate, bisphenol polycarbonate, poly(vinylidene di-fluouride) and polysulfone. The polymeric matrix was then decomposed using heat, leaving the particles to be characterized using transmission electron microscopy.
“These polymers spanned a variety of functional groups that differed in the strength and nature of their interactions with the iron oxide particles and in their position along with polymer chain,” Tannenbaum explained. “We found that the characteristic nanoparticle size decreased with the increasing affinity – the strength of the interaction – between the polymer and the iron oxide particles.”
Specifically, iron oxide particles formed in strongly interacting polymer media tended to be small (10-20 nanometers in diameter) and pyramid-shaped, while those formed in weakly-interacting media tended to be larger (40 to 60 nanometers in diameter) and spherical.
The researchers also found that the length of the polymer chain was only weakly related to the particle growth. “This means that for the same result, we can work in the melt with lower molecular-weight materials and have lower glass transitions,” Tannenbaum explained.
Based on the experimental results, Tannenbaum and Associate Professor Nily Dan of Drexel’s Department of Chemical Engineering charted the relationship between average particle size and the reactivity of the polymer interface. That information should help other scientists as they attempt regulate the growth of nanoparticles using polymer reactivity.
Tannenbaum and Dan theorize that the polymer layer surrounding a nanoparticle while it grows favors an optimal interfacial curvature that sets the equilibrium particle characteristics. That may be related to the free energy of the adsorbed polymer layer.
While the researchers focused on iron oxide in this work, they believe the control mechanism should be broadly applicable to other particles and polymeric materials.
Next, the researchers plan to explore the influence of polymers in solution – a more complicated task involving more variables.
“In solution, the situation is much more complicated,” Tannenbaum said. “The polymer chains are on the loose, and face competition from the solvent. The chains will be reluctant to adsorb onto the surface of the particles, so we may end up with larger particles than in the melt. In solution, molecular weight of the polymer will have an impact.”
The work reported at the American Chemical Society meeting is part of a broader study of how nanoparticles interact with polymers – specifically, the interface between polymer chains and nanoparticles.
“The interface has important fundamental properties,” Tannenbaum noted. “When you look at nanocomposites, the interface is a very large component of the whole structure. You can’t look at a nanocomposite as having just two components – the interface is really a third.”
Beyond their use as a means for controlling nanoparticle size, nanocomposites may also have applications of their own. Their periodic structure, for instance, can be useful in optical and photonic applications.
Media Contact
More Information:
http://www.edi.gatech.eduAll latest news from the category: Materials Sciences
Materials management deals with the research, development, manufacturing and processing of raw and industrial materials. Key aspects here are biological and medical issues, which play an increasingly important role in this field.
innovations-report offers in-depth articles related to the development and application of materials and the structure and properties of new materials.
Newest articles

Targeted use of enfortumab vedotin for the treatment of advanced urothelial carcinoma
New study identifies NECTIN4 amplification as a promising biomarker – Under the leadership of PD Dr. Niklas Klümper, Assistant Physician at the Department of Urology at the University Hospital Bonn…
A novel universal light-based technique
…to control valley polarization in bulk materials. An international team of researchers reports in Nature a new method that achieves valley polarization in centrosymmetric bulk materials in a non-material-specific way…

How evolution has optimised the magnetic sensor in birds
The magnetic sense of migratory birds is probably based on the protein cryptochrome 4, and a genetic study has now provided further support for this theory. A team of researchers…





















