Nanoparticles could aid biohazard detection, computer industry
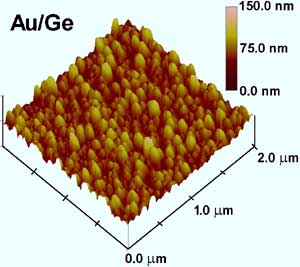
A micrograph image of gold nanoparticles form on germanium, an advanced semiconductor material. These tiny particles could create better connections between microchips and the much larger wires that lead to other computer components. (Graphic/Lon Porter) <br>
Nanotechnology could make life easier for computer manufacturers and tougher for terrorists, reports a Purdue University research team.
A group led by Jillian Buriak has found a rapid and cost-effective method of forming tiny particles of high-purity metals on the surface of advanced semiconductor materials such as gallium arsenide. While the economic benefits alone of such a discovery would be good news to chip manufacturers, who face the problem of connecting increasingly tiny computer chips with macro-sized components, the group has taken their research a step further.
The scientists also have learned how to use these nanoparticles as a bridge to connect the chips with organic molecules. Biosensors based on this development could lead to advances in the war on terrorism.
“We have found a way to connect the interior of a computer with the biological world,” said Buriak, associate professor of chemistry in Purdue’s School of Science. “It is possible that this discovery will enable chips similar to those found in computers to detect biohazards such as bacteria, nerve gas or other chemical agents.”
The research, which appears in today’s (Wednesday, 12/11) issue of Nano Letters, sprang from the team’s desire to attach metals to semiconductors in precise locations.
Computer chips, commonly made of silicon, contain circuits that are far smaller than those made from metal wires. But for an impulse from a keyboard or mouse to reach the microchip, the electronic signal must pass from a large wire onto the chip’s surface. The delicate interface between the macro and micro world is often accomplished by a tiny connection made of gold, chosen frequently over alternatives such as copper or silver, because it does not corrode in air. Gold’s advantages have made it the first choice for designers, though until now such advantages have come at a steep economic price.
“Gold works great once you actually get it onto the chip,” said Lon Porter, a chemistry graduate student in Buriak’s group. “But by traditional manufacturing methods you need to begin with expensive, very high-purity gold. With our method, however, you’d no longer need the high-quality gold you might find in coins in Fort Knox – you could use the low-purity gold waste swept up from the coin factory floor.”
In their purest forms, precious metals such as gold and platinum are among the most coveted substances in the world. But these metals are more commonly found in nature as part of low-purity compounds like metal salts – which, despite their name, are not salts you would use to flavor food or make a snowy roadway safe for driving. The amount of precious metals in these salts is low to begin with; when the salts are dissolved in liquid at the concentration that Buriak’s group needs to form nanoparticles, a test tube full of the solution is worth only pennies. But despite the low market value of the chemical solutions themselves, the effect Buriak’s group has discovered may nonetheless prove to be a gold mine.
“All you need to do to form nanoparticles is dip the semiconductor into the solution and wait,” Porter said. “Though you begin with a solution worth less than the change in your pocket, you still end up with a layer of gold nanoparticles on the silicon that has the same purity as gold bullion. Because the reaction sustains itself, manufacturers would not need any special training or equipment to use it. From both a manpower and a technical perspective, the process is a real money saver.”
The particles grow larger with increased time in the solution and eventually cover the semiconductor base with a bumpy coating. The roughness of the coating gives the base a greater surface area than it had by itself, a realization which led to the team’s second breakthrough.
“It’s similar to the way your brain packs a lot of surface area into the limited space inside your skull by folding in on itself many times,” Porter said. “But the advantage we found for computer chips is not that we can increase their ’thinking power,’ per se. Rather, the resulting rough surface gives us a lot of nooks and crannies in which to secure a second group of molecules atop the gold – organic molecules that react in the presence of other chemicals.”
The upshot of this double-layering is that the organic molecules could be chosen for their ability to react to the presence of nerve gas or biological contaminants. If a dangerous chemical reacted with an organic molecule, the metal nanoparticles could convey a signal downward to the chip that a biohazard was present.
“When a chemical reaction takes place, a small but measurable electrical change takes place,” Porter said. “As metals are excellent conductors of electricity, nanoparticles could be the bridge that we need to make computers interface with the biological world.”
Further refinement of their techniques has allowed the group to deposit nanoparticles of gold, platinum and other metals in specific areas of the semiconductor’s surface. Rather than a film that blankets the entire surface, the group can deposit the particles in a grid pattern or even draw lines with a microscopic “pen.” These refinements could allow manufacturers to put their discoveries to use comparatively rapidly.
“We are not sure what application of our discoveries will appear first,” Buriak said. “But there are many semiconductor companies out there that spend a lot of money on chip interfacing, and we expect they could all take advantage of this technique somehow.”
This research was funded in part by the National Science Foundation.
Buriak’s group is affiliated with Purdue’s new Birck Nanotechnology Center, scheduled for completion in the fall of 2004. A dozen groups associated with the center are pursuing research topics such as microscopic machines, advanced materials and artificial organs.
Writer: Chad Boutin, (765) 494-2081, cboutin@purdue.edu
Sources: Jillian M. Buriak, (765) 494-5302, buriak@purdue.edu
Lon A. Porter Jr., (765) 496-3491, porterjr@purdue.edu
Purdue News Service: (765) 494-2096; purduenews@purdue.edu
Electroless Nanoparticle Film Deposition Compatible with Photolithography, Microcontact Printing, and Dip-Pen Nanolithography Patterning Technologies
By Lon A. Porter, Jr., Hee Cheul Choi, J. M. Schmeltzer, Alexander E. Ribbe, Lindsay C. C. Elliott,
and Jillian M. Buriak*
Nanoparticles of Au, Pd and Pt form spontaneously as thin, morphologically complex metallic films upon various semiconducting or metal substrates such as Ge(100), Cu, Zn, and Sn, via galvanic displacement from aqueous metal salt solutions. Patterning of these high surface area metal films into ordered structures utilizing photolithography, microcontact printing (-CP), and dip-pen nanolithography (DPN) is demonstrated on flat Ge(100), and (for -CP) on rough Zn foil.
Media Contact
All latest news from the category: Materials Sciences
Materials management deals with the research, development, manufacturing and processing of raw and industrial materials. Key aspects here are biological and medical issues, which play an increasingly important role in this field.
innovations-report offers in-depth articles related to the development and application of materials and the structure and properties of new materials.
Newest articles

Microscopic basis of a new form of quantum magnetism
Not all magnets are the same. When we think of magnetism, we often think of magnets that stick to a refrigerator’s door. For these types of magnets, the electronic interactions…

An epigenome editing toolkit to dissect the mechanisms of gene regulation
A study from the Hackett group at EMBL Rome led to the development of a powerful epigenetic editing technology, which unlocks the ability to precisely program chromatin modifications. Understanding how…

NASA selects UF mission to better track the Earth’s water and ice
NASA has selected a team of University of Florida aerospace engineers to pursue a groundbreaking $12 million mission aimed at improving the way we track changes in Earth’s structures, such…