New regulations standardize optical coherence tomography

OCT device with image of the anterior segment of the eye.
© Heidelberg Engineering GmbH
Joint expertise from industry and research:
Optical coherence tomography (OCT) is a groundbreaking imaging technique that generates high-resolution cross-sectional images of transparent and semitransparent media. It is used both in industrial metrology, for example in quality assurance, and in biomedical diagnostics, such as ophthalmology or tissue diagnostics.
Companies and research institutes focusing on industrial metrology and medical technology also expect a wide range of other fields of application. They cooperate in the VDI/VDE – Society for Measurement and Automatic Control (GMA) in an expert committee on OCT. They have recently published the guideline VDI/VDE 5565 part 1 with standardized process descriptions.
The technical committee 1.32 “Optical Coherence Tomography” goal in GMA’s “Methodology of Measurement and Sensor Technology” division is to bring together expertise from industry and research and thus open the way for further OCT application fields in the future. The basis for using OCT in biomedical diagnostics and beyond is set by the standards published by the committee in January 2023.
So far, the comparatively new measurement method has not yet been described in a generally valid manner, and various procedures and performance characteristics have not yet been standardized. Thus, the comparability of different OCT systems was not given, and broader dissemination was limited. This is now to change through the standardization work of the technical committee.
“During the development of the technical regulations, we focused on the users and their specific requirements. The definition of terms and measurement methods or the description of typical variants of OCT methods creates an urgently needed uniformity. This is a basis for using OCT in different fields of application,” explains Niels König, head of the Production Measurement Technology department at the Fraunhofer Institute for Production Technology IPT in Aachen and chairman of the expert committee.
High-resolution imaging: enabler for multiple applications in biomedicine and beyond
Initially used in ophthalmology, OCT is a measurement technique based on low-coherence interferometry for non-contact and high-resolution imaging of tomographic cross-sectional images. It has a penetration depth of several millimeters and a resolving power in the single-digit micrometer range. In medical diagnostics, OCT can be used to examine organic tissue, such as tumor tissue at a very early stage of disease, either intraoperatively or in vitro. OCT is a technology that brings the potential to improve imaging in all medical disciplines – from minimally invasive surgery to neurosurgery and cardiology.
But thanks to its universal properties, OCT is also used in industry for material and defect testing, such as plastic products. In the case of opaque materials into which light cannot penetrate, for example metal, OCT is used for surface and distance measurement.
In January 2023, the guideline VDI/VDE 5565 Part 1 “Optical Coherence Tomography (OCT); Process Descriptions” was published in a bilingual edition (German/English) (www.vdi.de/5565). The guideline describes the OCT procedures and defines the necessary terms. The committee is already working on VDI/VDE 5565 Sheet 2 on “Signal processing and data evaluation”.
The guideline VDI/VDE 5565 part 1, “Optical Coherence Tomography (OCT) – Process descriptions,” can be ordered from 73.80 EUR at Beuth Verlag, www.beuth.de/en, (Phone: +49 30 2601-2260).
For further information, please contact the GMA office:
VDI/VDE Society
Measurement and Automatic Control (GMA)
Erik Marquardt
Phone +49 211 6214-373
marquardt@vdi.de
www.beuth.de/en – Search term: VDI/VDE 5565
Wissenschaftliche Ansprechpartner:
Dipl.-Phys. Niels König
Head of department “Production metrology”
Fraunhofer Institute for Production Technology IPT
Steinbachstr. 17
52074 Aachen, Germany
Phone +49 241 8904-113
niels.koenig@ipt.fraunhofer.de
Weitere Informationen:
https://www.ipt.fraunhofer.de/en/Press/Pressreleases/230505-new-regulations-stan…
Media Contact
All latest news from the category: Medical Engineering
The development of medical equipment, products and technical procedures is characterized by high research and development costs in a variety of fields related to the study of human medicine.
innovations-report provides informative and stimulating reports and articles on topics ranging from imaging processes, cell and tissue techniques, optical techniques, implants, orthopedic aids, clinical and medical office equipment, dialysis systems and x-ray/radiation monitoring devices to endoscopy, ultrasound, surgical techniques, and dental materials.
Newest articles
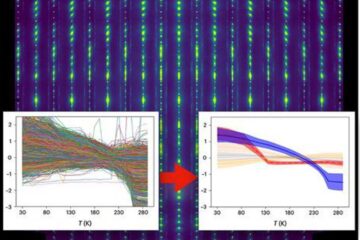
Machine learning algorithm reveals long-theorized glass phase in crystal
Scientists have found evidence of an elusive, glassy phase of matter that emerges when a crystal’s perfect internal pattern is disrupted. X-ray technology and machine learning converge to shed light…
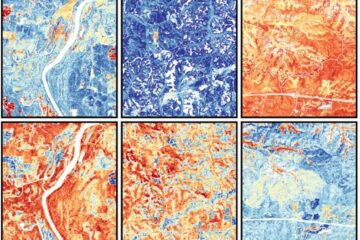
Mapping plant functional diversity from space
HKU ecologists revolutionize ecosystem monitoring with novel field-satellite integration. An international team of researchers, led by Professor Jin WU from the School of Biological Sciences at The University of Hong…

Inverters with constant full load capability
…enable an increase in the performance of electric drives. Overheating components significantly limit the performance of drivetrains in electric vehicles. Inverters in particular are subject to a high thermal load,…




















A guideline is not a “new regulation”.