Harnessing skin cancer genes to heal hearts
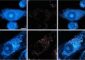
Two cross sections of engineered rat cardiac tissue showing the BRAF mutation at work. The BRAF-altered cell on the right has more newly synthesized DNA (green), showing the mutation is inducing cell division.
Credit: Nicholas Strash, Duke University
A common powerful mutation found in melanoma can push heart muscle cells to multiply in laboratory models of heart tissue.
Biomedical engineers at Duke University have demonstrated that one of the most dangerous mutations found in skin cancers might moonlight as a pathway to mending a broken heart.
The genetic mutation in the protein BRAF, a part of the MAPK signaling pathway that can promote cell division, is one of the most common and most aggressive found in melanoma patients. In a new study, researchers show that introducing this mutation to rat heart tissue grown in a laboratory can induce growth.
Repairing cardiac muscle after a heart attack is the “holy grail” of heart research, complicated by the fact that heart tissue does not regenerate on its own. One potential strategy would be to persuade heart muscle cells to divide by safely delivering a therapeutic gene to patients and fully controlling its activity in the heart.
The new study, appearing online January 24 in the journal Science Advances, lays important steps in finding ways to achieve this goal.
“Mature heart muscle cells do not typically divide, so we thought we’d need an especially strong genetic mutation to convince them to multiply,” said Nenad Bursac, professor of biomedical engineering at Duke. “MAPK is a well understood pathway that, when mutated, can be pretty aggressive at inducing proliferation in cancers, which is why we chose to look into it.”
In the study, Bursac and PhD student Nicholas Strash studied neonatal rat heart cells grown within a 3D hydrogel environment. Developed by the laboratory over more than a decade, the hydrogel environment provides the cues to grow and mature cells into adult-like heart muscle tissues, where cell division naturally stops.
Then, to try to get the muscle to divide and grow again, the researchers infected it with a virus loaded with a mutated BRAF gene. Following its normal behavior, the virus inserted the mutated gene into the cells, causing it to become a part of the cells’ DNA. The researchers then introduced a drug that caused the mutated BRAF genes to activate.
As with skin cancer, once activated, the mutant genes caused the heart muscle cells to enter DNA synthesis — the first step in cell division and growth. But there were also drawbacks.
“Once the cells started entering into their multiplication phase, they also began disassembling the machinery that allows them to contract and pump blood when in the heart,” Strash said. “It caused the tissue as a whole to lose about 70% of its contractile strength, which is pretty dramatic. One reason for this is that almost all cells in the tissue got infected by the virus.”
The results are both exciting and revealing. Any potential therapeutic that can get adult heart cells to proliferate is cause for optimism. But with the accompanying loss of strength, the dosage and duration of gene activation needs to be precisely controlled – thus there’s much to do before any potential use in human patients.
For starters, a different delivery system would have to be applied that can deliver the genes to the correct cells in a way that clinicians can fully control. Methods such as lipid nanoparticles and short-living viruses are two approaches currently being developed, but both still have a ways to go before they could be applied for heart regeneration in humans.
The other major hurdle is finding a way to jump start heart tissue regeneration without causing the tissue to lose strength. Based on the timing of cellular mechanisms, the researchers believe there may be a window in which the mutated gene activity could be stopped after the replication begins, but before the contractile machinery is affected in a large portion of the heart. Or there may be an opportunity to administer a second therapeutic that could prompt the cells to rebuild the dismantled pumping machinery after proliferation.
Moving forward, the researchers are planning to study how this approach works in the hearts of live animals and compare it to their lab-tested results. Working with live animals will also provide a better understanding of what other genes and processes are activated by the mutated BRAF gene, and whether proliferation could be independently activated without functional decline to efficiently promote healing.
“The heart essentially does not have primary cancers, and it’s almost unique that it doesn’t,” Bursac said. “Introducing this cancer mutation in the heart is obviously an engineered outcome that doesn’t happen naturally. Studying it in lab-grown tissues is a great step toward understanding what this entire signaling pathway does within the heart, which could have benefits beyond regenerative therapies.”
The research was supported by the National Institutes of Health (U01HL134764, R01HL164013, 5T32HD040372, 1F31HL156453, 1F31HL162460), the Translating Duke Health Initiative and a Foundation Leducq grant (15CVD03).
CITATION: “Time-dependent Effects of BRAF-V600E on Cell Cycling, Metabolism, and Function in Engineered Myocardium,” Nicholas Strash, Sophia DeLuca, Geovanni L. Janer Carattini, Yifan Chen, Tianyu Wu, Abbigail Helfer, Jacob Scherba, Isabella Wang, Mehul Jain, Ramona Naseri, and Nenad Bursac. Science Advances, Jan. 24 2024. DOI: 10.1126/sciadv.adh2598
Journal: Science Advances
DOI: 10.1126/sciadv.adh2598
Method of Research: Experimental study
Subject of Research: Cells
Article Title: Time-dependent Effects of BRAF-V600E on Cell Cycling, Metabolism, and Function in Engineered Myocardium
Article Publication Date: 24-Jan-2024
Media Contact
Ken Kingery
Duke University
ken.kingery@duke.edu
Office: 919-660-8414
Original Source
All latest news from the category: Medical Engineering
The development of medical equipment, products and technical procedures is characterized by high research and development costs in a variety of fields related to the study of human medicine.
innovations-report provides informative and stimulating reports and articles on topics ranging from imaging processes, cell and tissue techniques, optical techniques, implants, orthopedic aids, clinical and medical office equipment, dialysis systems and x-ray/radiation monitoring devices to endoscopy, ultrasound, surgical techniques, and dental materials.
Newest articles

A universal framework for spatial biology
SpatialData is a freely accessible tool to unify and integrate data from different omics technologies accounting for spatial information, which can provide holistic insights into health and disease. Biological processes…

How complex biological processes arise
A $20 million grant from the U.S. National Science Foundation (NSF) will support the establishment and operation of the National Synthesis Center for Emergence in the Molecular and Cellular Sciences (NCEMS) at…

Airborne single-photon lidar system achieves high-resolution 3D imaging
Compact, low-power system opens doors for photon-efficient drone and satellite-based environmental monitoring and mapping. Researchers have developed a compact and lightweight single-photon airborne lidar system that can acquire high-resolution 3D…