
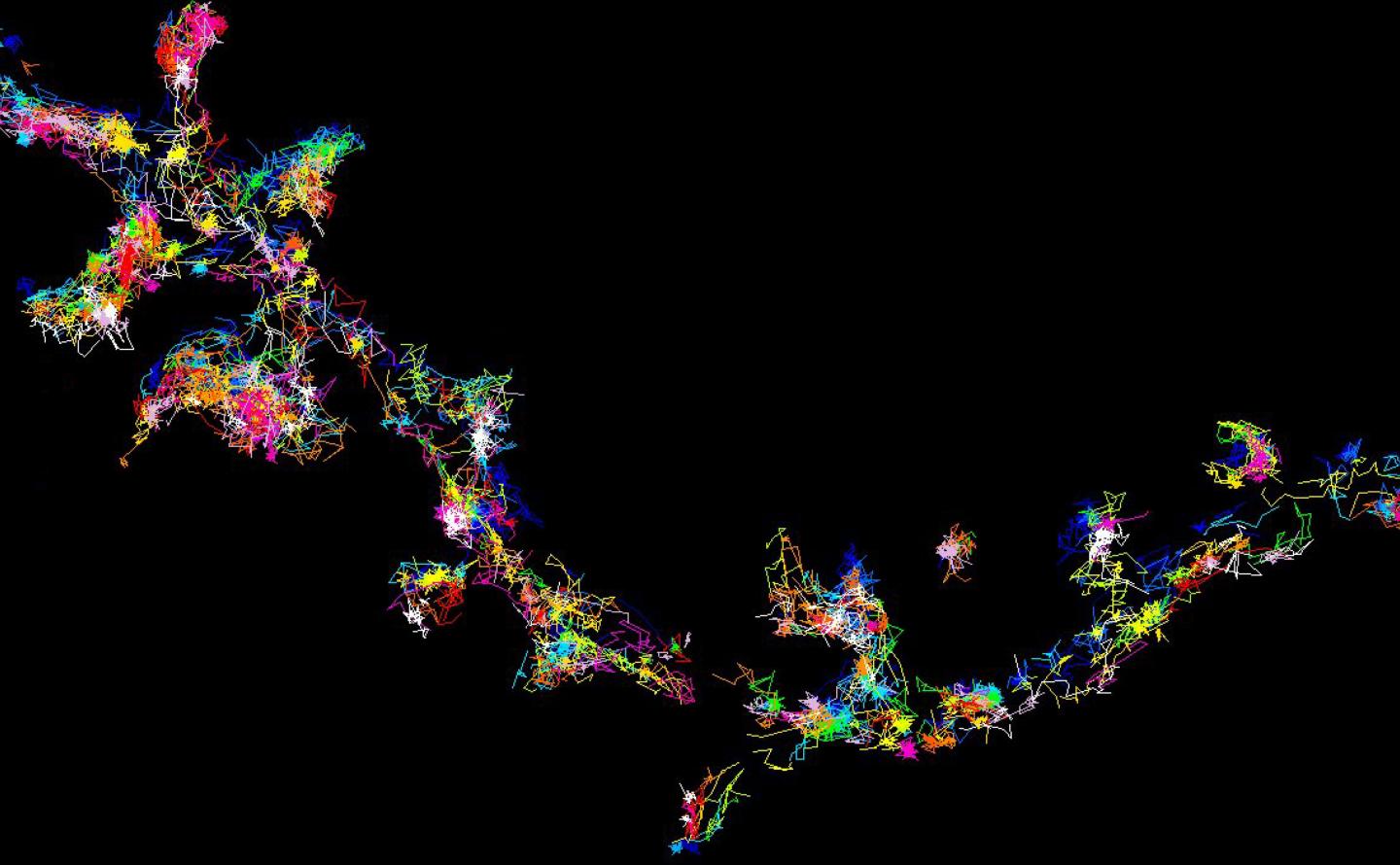
The signalling protein Fyn moving and forming clusters in living brain cells - viewed using super-resolution microscopy.
Credit: Meunier Lab, University of Queensland
UQ Queensland Brain Institute's Clem Jones Centre for Ageing and Dementia Research Professors Frédéric Meunier and Jürgen Götz found a protein, Tau, involved in Alzheimer's disease affects the organisation of the signalling protein Fyn, which plays a critical role in memory formation.
“One of the distinguishing features of Alzheimer's disease is the tangles of Tau protein that form inside brain cells, but this is the first time anyone has demonstrated that Fyn nanoclustering is affected by Tau,” Professor Götz said.
Professor Meunier said single molecule imaging in living brain cells allowed unprecedented access to the organisation of key proteins in small nanoclusters that were not detectable previously.
“We have shown that Tau controls the Fyn nanoclustering in dendrites, where the communication between brain cells occurs,” Professor Meunier said.
“When Tau is mutated, Fyn makes aberrantly large clusters, thereby altering nerve signals and contributing to dysfunction of the synapse-junctions between nerve cells.”
Professor Meunier's team used the super-resolution single molecule imaging technique to see how Tau and its mutants control Fyn nanoclustering.
Professor Meunier went on to investigate a different mutant of Tau found in families with a very high risk of developing frontotemporal dementia and found that Fyn was over-clustered in the spines of dendrites.
“Imagine that you have clustering of Fyn, a signalling molecule, throughout your life; it's going to give rise to an over-signalling problem — this could be one of the ways in which Fyn is toxic to cells,” he said.
“The spines of the dendrites are critical to how nerve cells communicate with each other and underpin memory and learning.”
Exactly what causes Alzheimer's and other forms of dementia is still a mystery, but Fyn is linked to both the plaques of amyloid protein that form between brain cells, and tangles of Tau protein that form inside brain cells — two distinguishing features of Alzheimer's disease.
“Super-resolution single molecule imaging gives us an unprecedented insights into what is happening in living nerve cells, with the aim of understanding the biology behind these complex and debilitating diseases,” Professor Meunier said.
###
The study was published in the journal eLife and supported by organisations including the Australian and Queensland governments, the National Health and Medical Research Council of Australia and the Australian Research Council.












