Researchers develop new tools to rapidly test activity of anti-coronavirus antibodies
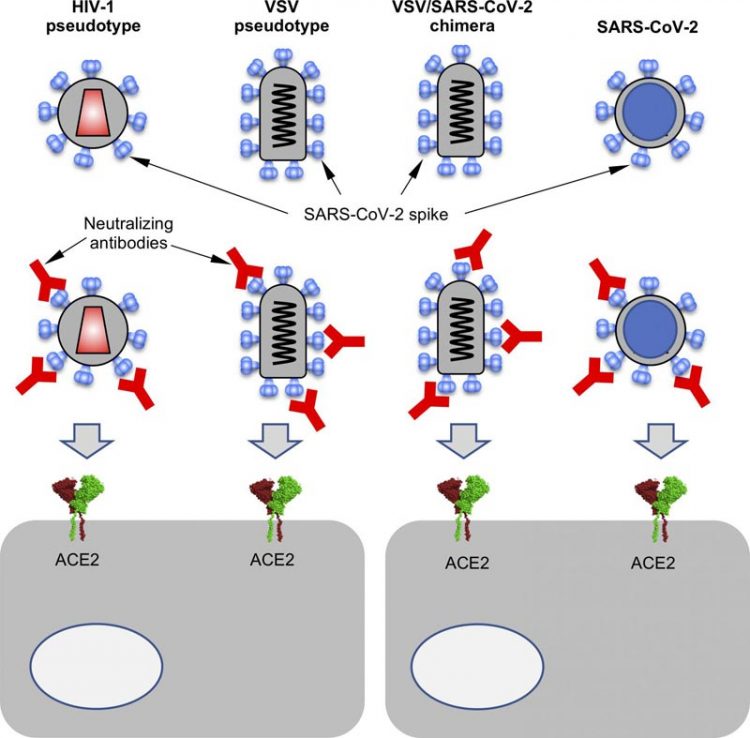
A diagram of how surrogate viruses expressing the SARS-CoV-2 spike protein can be used to measure the activity of neutralizing antibodies that target the spike protein and prevent the virus from entering cells. Credit: Schmidt et al., 2020 Usage Restrictions: Reporters may freely use these materials in news coverage with the appropriate credit information.
People infected with SARS-CoV-2 produce neutralizing antibodies that prevent the virus from infecting cells by binding to the spike protein on the virus's surface.
Early studies have suggested that the strength of this antibody response varies greatly between patients, and it remains unknown how long any such neutralizing antibodies persist in the blood to provide protection against reinfection.
Meanwhile, efforts are under way to treat and prevent COVID-19 using either purified antibodies or whole blood plasma collected from convalescent patients who produce large amounts of neutralizing antibodies. Moreover, any successful vaccine against SARS-CoV-2 will have to induce the production of neutralizing antibodies.
“Whether elicited by natural infection or vaccination, or administered as convalescent plasma or in recombinant form, neutralizing antibodies will likely be crucial for curtailing the global burden of COVID-19 disease,” says Paul D. Bieniasz, a professor at The Rockefeller University and investigator at the Howard Hughes Medical Institute.
“For this reason, the availability of rapid, convenient, and accurate assays that measure neutralizing antibody activity is crucial for evaluating naturally acquired or artificially induced immunity against SARS-CoV-2.”
Antibody tests using the SARS-CoV-2 virus itself are labor intensive and must be carried out in biosafety level 3 facilities, limiting their widespread application. Bieniasz and colleagues therefore developed a number of safer, surrogate viruses that can be used in place of SARS-CoV-2 to test the neutralizing activity of antibodies targeting the coronavirus spike protein.
The surrogate viruses are versions of either the human immunodeficiency virus type-1 (HIV-1) or vesicular stomatitis virus (VSV) that produce the SARS-CoV-2 spike protein instead of their own surface proteins. Some of these surrogate viruses are unable to replicate, making them even safer to use in the laboratory.
Moreover, the viruses are engineered to generate fluorescent or luminescent infected cells, making it easy for researchers to track infection and measure how well this process is blocked by potential neutralizing antibodies.
Bieniasz and colleagues tested the ability of convalescent patient plasma samples and purified antibodies to block the entry of the surrogate viruses into human cells grown in the laboratory.
“Each of the surrogate virus-based assays generated quantitative measurements of neutralizing activity that correlated well with neutralization measured using authentic SARS-CoV-2,” says Theodora Hatziioannou, a research associate professor at Rockefeller who co-directed the study with Bieniasz.
“In just a few weeks, we have already used these assays to determine the neutralizing potencies of hundreds of plasma samples and monoclonal antibodies in a biosafety level 2 laboratory.”
Bieniasz adds, “Automation and additional miniaturization is certainly feasible to further increase throughput–a notable consideration given the sheer number of vaccine candidates in the development pipeline. We think that these surrogate viruses and assays will be of significant use in curtailing the COVID-19 pandemic.”
###
Schmidt et al., 2020. J. Exp. Med. https:/
About the Journal of Experimental Medicine The Journal of Experimental Medicine (JEM) features peer-reviewed research on immunology, cancer biology, stem cell biology, microbial pathogenesis, vascular biology, and neurobiology. All editorial decisions are made by research-active scientists in conjunction with in-house scientific editors. JEM makes all of its content free online no later than six months after publication. Established in 1896, JEM is published by Rockefeller University Press. For more information, visit http://rupress.org/jem/>jem.org.
Visit our http://rupress.org/newsroom>Newsroom, and sign up for a weekly preview of articles to be published. Embargoed media alerts are for journalists only.
Follow JEM on Twitter at https://twitter.com/jexpmed>@JExpMed and https://twitter.com/rockupress>@RockUPress.
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Properties of new materials for microchips
… can now be measured well. Reseachers of Delft University of Technology demonstrated measuring performance properties of ultrathin silicon membranes. Making ever smaller and more powerful chips requires new ultrathin…

Floating solar’s potential
… to support sustainable development by addressing climate, water, and energy goals holistically. A new study published this week in Nature Energy raises the potential for floating solar photovoltaics (FPV)…

Skyrmions move at record speeds
… a step towards the computing of the future. An international research team led by scientists from the CNRS1 has discovered that the magnetic nanobubbles2 known as skyrmions can be…





















