First direct look at how light excites electrons to kick off a chemical reaction
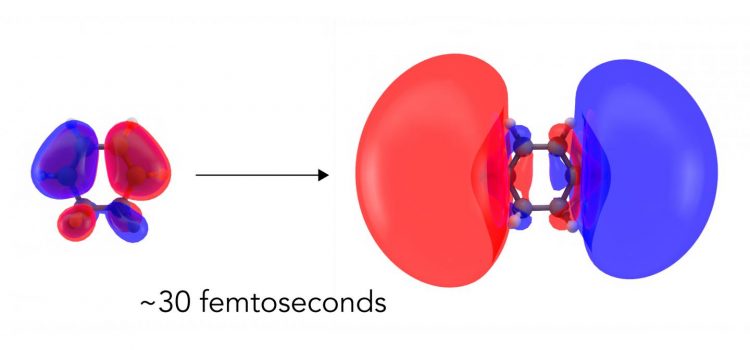
Scientists have directly seen the first step in a light-driven chemical reaction for the first time. They used an X-ray free-electron laser at SLAC to capture nearly instantaneous changes in the distribution of electrons when light hit a ring-shaped molecule called CHD. Within 30 femtoseconds, or millionths of a billionth of a second, clouds of electrons deformed into larger, more diffuse clouds corresponding to an excited electronic state. Credit: Greg Stewart/SLAC National Accelerator Laboratory
The first step in many light-driven chemical reactions, like the ones that power photosynthesis and human vision, is a shift in the arrangement of a molecule's electrons as they absorb the light's energy. This subtle rearrangement paves the way for everything that follows and determines how the reaction proceeds.
Now scientists have seen this first step directly for the first time, observing how the molecule's electron cloud balloons out before any of the atomic nuclei in the molecule respond.
While this response has been predicted theoretically and detected indirectly, this is the first time it's been directly imaged with X-rays in a process known as molecular movie-making, whose ultimate goal is to observe how both electrons and nuclei act in real time when chemical bonds form or break.
Researchers from Brown University, the University of Edinburgh and the Department of Energy's SLAC National Accelerator Laboratory reported their findings in Nature Communications today.
“In past molecular movies, we have been able to see how atomic nuclei move during a chemical reaction,” said Peter Weber, a chemistry professor at Brown and senior author of the report. “But the chemical bonding itself, which is a result of the redistribution of electrons, was invisible. Now the door is open to watching the chemical bonds change during reactions.”
A model for important biological reactions
This was the latest in a series of molecular movies starring 1,3-cyclohexadiene, or CHD, a ring-shaped molecule derived from pine oil. In a low-pressure gas its molecules float freely and are easy to study, and it serves as an important model for more complex biological reactions like the one that produces vitamin D when sunlight hits your skin.
In studies going back almost 20 years, scientists have studied how CHD's ring breaks apart when light hits it – first with electron diffraction techniques, and more recently with SLAC's “electron camera,” MeV-UED, and X-ray free-electron laser, the Linac Coherent Light Source (LCLS). These and other studies around the world have revealed how the reaction proceeds in finer and finer detail.
Four years ago, researchers from Brown, SLAC and Edinburgh used LCLS to make a molecular movie of the CHD ring flying apart, – the first-ever molecular movie recorded using X-rays. This achievement was listed as one of the 75 most important scientific breakthroughs to emerge from a DOE national laboratory, alongside discoveries such as the decoding of DNA and the detection of neutrinos.
But none of those previous experiments were able to observe the initial electron-shuffling step, because there was no way to tease it apart from the much larger movements of the molecule's atomic nuclei.
Electrons in the spotlight
For this study, an experimental team led by Weber took a slightly different approach: They hit samples of CHD gas with a wavelength of laser light that excited the molecules into a state that lives for a relatively long period of time – 200 femtoseconds, or millionths of a billionth of a second – so their electronic structure could be probed with LCLS X-ray laser pulses.
“X-ray scattering has been used to determine the structure of matter for more than 100 years,” said Adam Kirrander, a senior lecturer at Edinburgh and senior co-author of the study, “but this is the first time the electronic structure of an excited state has been directly observed.”
The technique used, called non-resonant X-ray scattering, measures the arrangement of electrons in a sample, and the team hoped to capture changes in the distribution of electrons as the molecule absorbed the light. Their measurement bore out that expectation: While the signal from the electrons was weak, the researchers were able to unambiguously capture how the electron cloud deformed into a larger, more diffuse cloud corresponding to an excited electronic state.
It was critical to observe these electronic changes before the nuclei started moving.
“In a chemical reaction, the atomic nuclei move and it's difficult to disentangle that signal from the other parts that belong to chemical bonds forming or breaking,” said Haiwang Yong, a PhD student at Brown University and lead author of the report. “In this study, the change in the positions of atomic nuclei is comparatively small on that timescale, so we were able to see the motions of electrons right after the molecule absorbs light.”
SLAC senior staff scientist Michael Minitti added, “We're imaging these electrons as they move and shift around. This paves the way to watching electron motions in and around bond breaking and bond formation directly and in real time; in that sense it's similar to photography.”
###
Nikola Zotev, a PhD student in Kirrander's lab at the University of Edinburgh, played a leading role in computational and modeling work for the study. LCLS is a DOE Office of Science user facility, and major funding for this research came from the Office of Science and the Carnegie Trust for the Universities of Scotland.
Citation: Haiwang Yong et al., Nature Communications, 1 May 2020 (10.1038/s41467-020-15680-4)
SLAC is a vibrant multiprogram laboratory that explores how the universe works at the biggest, smallest and fastest scales and invents powerful tools used by scientists around the globe. With research spanning particle physics, astrophysics and cosmology, materials, chemistry, bio- and energy sciences and scientific computing, we help solve real-world problems and advance the interests of the nation.
SLAC is operated by Stanford University for the U.S. Department of Energy's Office of Science. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time.
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Superradiant atoms could push the boundaries of how precisely time can be measured
Superradiant atoms can help us measure time more precisely than ever. In a new study, researchers from the University of Copenhagen present a new method for measuring the time interval,…

Ion thermoelectric conversion devices for near room temperature
The electrode sheet of the thermoelectric device consists of ionic hydrogel, which is sandwiched between the electrodes to form, and the Prussian blue on the electrode undergoes a redox reaction…

Zap Energy achieves 37-million-degree temperatures in a compact device
New publication reports record electron temperatures for a small-scale, sheared-flow-stabilized Z-pinch fusion device. In the nine decades since humans first produced fusion reactions, only a few fusion technologies have demonstrated…





















