
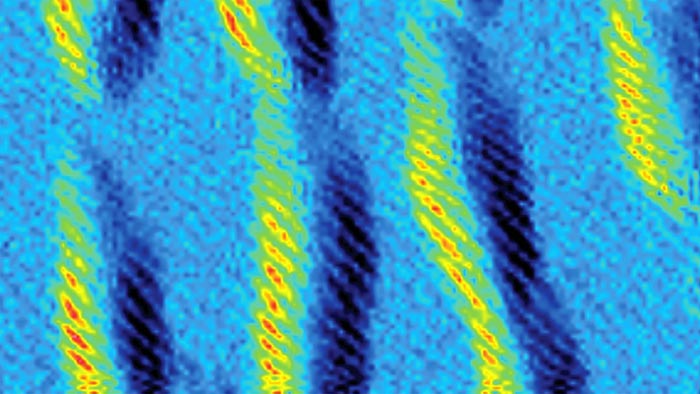
These spring-like structures are helix-shaped polymers. The helical coils are indicated by bright stripes in this side view of the tiny molecule-sized threads laying on a surface. A team of chemists used click chemistry to build these SOF4-based polymers. This image was taken by atomic force microscopy.
Credit: Han Zuilhof lab
SOF4 is a gas that was discovered over a hundred years ago but is rarely used because it is difficult to prepare and highly reactive. Now a collaboration of chemists including Cold Spring Harbor Laboratory (CSHL) Professor John E. Moses, Nobel laureate K. Barry Sharpless and Associate Professor Peng Wu, both of Scripps Research, and Han Zuilhof of Wageningen University found a way to use the molecule safely as building blocks for new products. In a paper in Nature Chemistry, they describe a new set of modifiable polymers made from SOF4.
It was a team effort to tame the molecule. Zuilhof says, “The goals of the various research groups were different, but we combined all our expertise to achieve our many goals.” Suhua Li, the lead author of the study and a former postdoc in Sharpless’ lab, started with a tiny container of the gas in the lab, but after he used that up, he says, “there was no more SOF4 available anywhere in the world. The great potential of the SOF4 chemistry inspired me to make the gas by myself, even though the procedure seemed dangerous.”
The SOF4 molecule could be used as a hub to link together diverse components into a modular family of new—and potentially valuable—materials. Even more importantly, the scientists found that the reactions could be done in an environmentally safe way without dangerous solvents and polluting byproducts—a form of what chemists call “click chemistry”.
Click chemistry allows users to “click” together two molecules with precision, speed, and reliability. The team used SOF4 to form long chains—polymers—with room for important modifications. Moses says:
“That’s why click chemistry is great, really! These polymers could be made in one day. As long as we have the gas, we could do all that chemistry in one day, make a polymer, and post-modify it in one day. That’s incredibly fast.”
This new chemistry will allow scientists to generate a vast new library of polymers, each with its own distinct properties and applications in drug discovery and material science. Sharpless says, “It is the making and breaking of the nascent, growing polymer links that let us access what seems magical.” Moses adds:
“The opportunity for these polymers, I think, is infinite. There are so many things we can do with it, really. We’re limited by our imagination.”
Journal: Nature Chemistry
DOI: 10.1038/s41557-021-00726-x
Article Title: “SuFExable polymers with helical structures derived from thionyl tetrafluoride”
Article Publication Date: 16-Aug-2021
Media Contact
Sara Roncero-Menendez
roncero@cshl.edu
Office: 516-367-6866












