Quantum Coherent-Like State Observed in a Biological Protein for the First Time
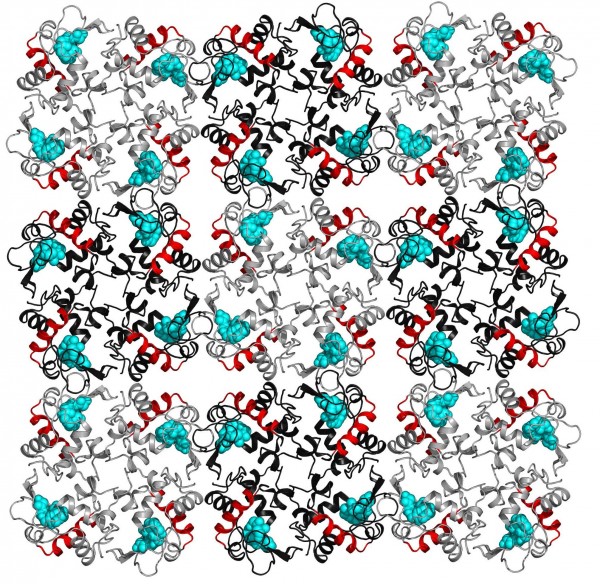
Gergely Katona, et al. Lysozyme molecules arranged in a crystal lattice. The red helical structures are associated with electron density changes when the protein crystal was exposed to terahertz radiation.
If you take certain atoms and make them almost as cold as they possibly can be, the atoms will fuse into a collective low-energy quantum state called a Bose-Einstein condensate. In 1968 physicist Herbert Fröhlich predicted that a similar process at a much higher temperature could concentrate all of the vibrational energy in a biological protein into its lowest-frequency vibrational mode. Now scientists in Sweden and Germany have the first experimental evidence of such so-called Fröhlich condensation.
The researchers made the condensate by aiming terahertz radiation at a crystallized protein extracted from the white of a chicken egg. They report their results in the journal Structural Dynamics, from AIP Publishing and the American Crystallographic Association.
“Observing Fröhlich condensation opens the door to a much wider-ranging study of what terahertz radiation does to proteins,” said Gergely Katona, a senior scientist at the University of Gothenburg in Sweden. Terahertz radiation occupies the space in the electromagnetic spectrum between microwaves and infrared light. It has been proposed as a useful tool in applications ranging from airport security to cancer screening, but its effects on biological systems remains murky.
Katona said he is interested in studying how terahertz-induced Fröhlich condensation could change the rates of reactions catalyzed by biological enzymes or shift chemical equilibria. Such knowledge could lead to medical applications or new ways to control chemical reactions in industry, but Katona cautioned that the research is still at a fundamental stage.
As far as the safety implications for terahertz radiation, Katona said that the jury is still out. The effects he and his team observed are reversible and last only for a short time, he added.
The Long Path from Theory to Observation
The theoretical underpinnings of Fröhlich condensation are relatively simple, Katona noted. Fröhlich proposed that when proteins absorb a terahertz photon the added energy forces the oscillating molecules into a single, lowest-frequency mode. In contrast, other models predict that the protein will quickly dissipate the energy from the photon in the form of heat.
To distinguish between the two outcomes, Katona and his colleagues turned to a technique called X-ray crystallography. The technique works by irradiating a sample with X-rays. By studying how the X-rays scatter and interfere with each other, scientists can work out the relative density of electrons in different locations in the sample material, which can then be used to tell the position of atoms and molecules.
For their protein, the researchers chose the enzyme lysozyme, which is a common immune system protein that attacks the cell walls of invading bacteria. Previous studies had indicated that low-frequency vibrations (in the terahertz domain) strongly influence the function of the protein.
Katona and his colleagues aimed short bursts of 0.4 terahertz radiation at the lysozyme crystals while simultaneously gathering X-ray crystallography data. The researchers separated the data gathered when the terahertz radiation was on from the data gathered when it was off, and then statistically analyzed each set. They found evidence that one of the helix structures in the protein was compressed during terahertz radiation, and that the compression lasted on the order of micro- to milli-seconds, thousands of times longer than could be explained by the thermal dissipation model. The researchers concluded that the long-lasting structural changes could only be explained by Fröhlich condensation, a quantum-like collective state in which the molecules in a protein behave as one.
The real-world support for Fröhlich's theory took so long to obtain because of the technical challenges of the experiment, Katona said.
“Terahertz radiation used to be very difficult to produce and is still difficult to handle,” he noted. Analyzing the X-ray data is also particularly challenging for low frequency vibrations, which are small in magnitude compared to typical structural changes in proteins.
The most important aspect of the experiment is that the researchers were able to detect structural changes in a protein that were not caused by heat dissipation, Katona said. Now that they've made the observations, the researchers are eager to explore how the structural changes affect the way the proteins work, he said.
The article, “Terahertz radiation induces non-thermal structural changes associated with Frohlich condensation in a protein crystal,” is authored by Ida V. Lundholm, Helena Rodilla, Weixiao Y. Wahlgren, Annette Duelli, Gleb Bourenkov, Josip Vukusic, Ran Friedman, Jan Stake, Thomas Schneider and Gergely Katona. It will be published in the journal Structural Dynamics on October 13, 2015 (DOI: 10.1063/1.4931825). After that date, it can be accessed at: http://scitation.aip.org/content/aip/journal/sdy/2/5/10.1063/1.4931825 .
The authors of this paper are affiliated with the University of Gothenburg, the Chalmers University of Technology, the European Molecular Biology Laboratory Hamburg Outstation and Linnaeus University.
ABOUT THE JOURNAL
Structural Dynamics is a journal devoted to research on the methods, techniques and understanding of time-resolved changes in chemical, biological and condensed matter systems. http://sd.aip.org
Contact Information
Jason Socrates Bardi
jbardi@aip.org
240-535-4954
@jasonbardi
Media Contact
All latest news from the category: Physics and Astronomy
This area deals with the fundamental laws and building blocks of nature and how they interact, the properties and the behavior of matter, and research into space and time and their structures.
innovations-report provides in-depth reports and articles on subjects such as astrophysics, laser technologies, nuclear, quantum, particle and solid-state physics, nanotechnologies, planetary research and findings (Mars, Venus) and developments related to the Hubble Telescope.
Newest articles

Silicon Carbide Innovation Alliance to drive industrial-scale semiconductor work
Known for its ability to withstand extreme environments and high voltages, silicon carbide (SiC) is a semiconducting material made up of silicon and carbon atoms arranged into crystals that is…

New SPECT/CT technique shows impressive biomarker identification
…offers increased access for prostate cancer patients. A novel SPECT/CT acquisition method can accurately detect radiopharmaceutical biodistribution in a convenient manner for prostate cancer patients, opening the door for more…

How 3D printers can give robots a soft touch
Soft skin coverings and touch sensors have emerged as a promising feature for robots that are both safer and more intuitive for human interaction, but they are expensive and difficult…





















