
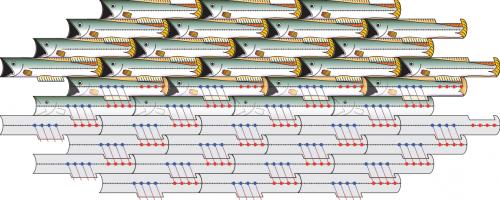
Simple shapes, such as these fish, can tile large surfaces if their geometry allows for symmetry. The edges each tessellating fish share with their surroundings are identical from fish to fish. Similarly, assemblies of collagen protein "tiles" can be achieved when the chemical and physical environments of every tile are designed to be identical, allowing scientists to produce synthetic collagen nanofibers.
Credit: Submitted University of Wisconsin-Madison
Much of the clinical supply comes from animals like pigs and cows, but it can cause allergic reactions or illness in some people. Functional human collagen has been impossible to create in the lab. Now, in a study published this month in Nature Chemistry, a team of University of Wisconsin-Madison researchers describe what may be the key to growing functional, natural collagen fibers outside of the body: symmetry.
“These are massive protein chains and it's difficult to make them,” says study lead author I. Caglar Tanrikulu, an assistant scientist in the laboratory of Ronald Raines, professor of chemistry and Henry Lardy Professor of Biochemistry. “You can't synthesize them chemically because they're really long. You can't make them biologically because of post-translational modifications,” the cellular-level touches that render collagen functional.
In the body, collagen is built by a process involving the interaction of three separate strands of collagen protein that intertwine to create a long, rope-like fiber, known as a triple helix.
For years, scientists have tried to get short pieces of collagen arranged into a triple helix to grow into these long fibers, but the process relies on chemistry and physical principles that are more complex than the relatively simpler rules of other molecules like DNA, which forms a double helix. So far, they have not succeeded in creating fibers that are either long enough or thick enough to mimic what is found in the body.
However, the work of these scientists has helped to establish some ground rules that govern the basic principles of collagen building. For example, they have found that making collagen requires having just the right amount of chemical and physical contact between individual strands to encourage them to fit together and grow.
Using the knowledge from new studies as a basic scaffold, Tanrikulu got to work “designing” collagen based on the rules he knew to be true. Those rules include a particular arrangement of amino acids — which create the collagen protein — and specific interactions between charged molecules on the individual collagen strands, called salt bridges, that help link them together.
A relative outsider to the field, Tanrikulu “naively” began to assemble a collection of possible designs, ignoring some of the dogma his more experienced colleagues had adopted in their work.
“My naiveté ended up being my biggest strength,” says Tanrikulu. “I looked at it in a way other people hadn't.”
He realized that each individual strand in the three-member collagen fiber had to “see” the exact same environment while leaving enough overlap between them that they could join up with other short pieces of collagen.
“All parts of the collagen fiber have to be experiencing the same thing,” Tanrikulu says. “Like floor tiles, if you know the shape of a tile and what symmetry to use, you can cover the whole surface; either every tile is arranged the same as the others or the edges won't fit. Similarly, if the ends of the growing collagen fiber are not regularly-spaced, the chemical environment won't be the same.”
Tanrikulu came up with multiple designs that fit the symmetry criteria. None of his predecessors' versions met it.
“The key is not making the peptide (the short version of a protein that serves as the individual tile in a larger molecule),” he says. “It's knowing which peptide to make.”
Using the rules and subsequent designs, Tanrikulu was able to grow long, stable collagen fibers in the laboratory that mimic those found in nature. Now, he is exploring ways to bring the findings to the world of biomaterials and nanotechnology, and is looking to collaborate with other scientists with expertise in these fields.
The lessons he has learned about the importance of symmetry in building collagen, he believes, could have implications for other types of molecules, which could be particularly informative for nanotechnology. And, equipped with a better way to grow molecules in the lab, he's hopeful it will have human health applications, too, even if that is far in the future.
“This is something that has never been done before. Will it have an eventual application? I hope so,” Tanrikulu says. “But that's not how science works.”
###
Kelly April Tyrrell, kelly.tyrrell@wisc.edu, 608-262-9772
This work was funded by the National Institutes of Health under award numbers R01EB008722, R01HL115553 and P30 AR050950.












