Penn study solves mystery of cell powerhouse's balance of calcium
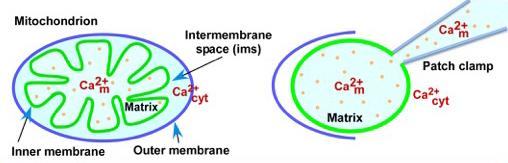
The mitochondrial matric calcium ion concentration regulates activity of the major mitochondrial calcium influx pathway, as measured by patch clamp physiology. Credit: The lab of Kevin Foskett, PhD, Perelman School of Medicine, University of Pennsylvania
A decades-long mystery of how the cell's powerhouse, and its energy currency of calcium ion flow, is maintained under different physiological conditions has been solved by researchers from the Perelman School of Medicine at the University of Pennsylvania.
The team, led by Kevin Foskett, PhD, chair of the department of Physiology, identified a novel regulatory mechanism that governs levels of calcium inside cells. Without this physiological mechanism, calcium levels can increase uncontrollably, contributing to a variety of neurodegenerative, metabolic, and cardiovascular diseases.
The findings, reported early online this month in Cell Reports, add important new insights into the gatekeeping mechanism of calcium entry into the cell power unit, called the mitochondria, and may help scientists better understand and target newly identified molecular components that regulate calcium flux.
“Understanding the molecular mechanisms by which mitochondrial calcium levels are regulated may have important implications for designing therapeutic targets for a variety of diseases, including diabetes, stroke, cancer, and age-related neurological diseases that have been related to mitochondrial dysfunction,” Foskett said. Mitochondria are comprised of two membranes. The outer membrane covers this cell component like a skin, and the inner membrane folds over many times, creating layers to increase surface area for the chemical reactions that produce the body's energy molecules. Disorders of mitochondria can disrupt energy production, essentially like an electrical brown out or black out.
Calcium is an important chemical messenger that regulates a variety of cellular processes. When calcium levels rise in the cell's interior during cell signaling, mitochondria rapidly take it in through a protein complex called the mitochondrial calcium uniporter (MCU). The MCU is an ion channel that governs uptake of calcium ions. Maintaining correct levels of calcium in and outside of the mitochondria is important because it is required for cellular energy production but an overload can lead to cell death.
Horia Vais, PhD, a senior research investigator in the Foskett lab measured calcium ion currents flowing through the MCU. He discovered that the concentration of calcium inside the mitochondria matrix strongly regulates the activity of MCU. The matrix contains enzymes, strands of DNA, protein crystals, glycogen, and lipid and occupies the inner space inside the mitochondria.
This mechanism ensures that MCU activity is low, preventing calcium overload inside the mitochondria. This gatekeeping brake can be overcome by higher matrix calcium concentrations during cell signaling. In 2012, the Foskett group and Temple University collaborators established in a seminal study published in Cell that the mitochondrial protein MICU1 is required to set the proper level of calcium uptake under normal conditions. However, the current study showed that MICU1 is not localized in the matrix, but in the inter-membrane space.
The authors established that one end of an MCU-associated membrane, called EMRE, resided in the mitochondrial matrix and contained acidic amino acids resembling calcium-sensing regions of other ion channels. Neutralizing these regions completely abolished calcium regulation, and the mitochondria became overloaded with calcium.
From this, the team found that EMRE-dependent matrix calcium regulation of MCU required MICU1, MICU2, and calcium on the other side of the inner membrane to work properly. EMRE couples calcium sensors on both sides of the inner membrane to regulate MCU activity and the extent of mitochondrial calcium flux. “We now know that this important ion channel gateway deep inside the cell is regulated by two gatekeepers, governed by EMRE,” Foskett said.
“Our study unravels the mystery of the mitochondrial gatekeeping mechanism,” said co-first author Karthik Mallilankaraman, PhD, a postdoctoral fellow in the Foskett lab who is now an assistant professor of Physiology at the National University of Singapore. “We have shown that mitochondria are protected from calcium overload by components on either side of the mitochondrial inner membrane — MICU proteins on one side and matrix calcium on the other — coupled by EMRE.”
###
Other authors, all from the Foskett lab, are Daniel Mak, Henry Hoff, Riley Payne, and Jessica Tanis. This work was supported by the National Institute of General Medical Sciences (GM56328).
Media Contact
All latest news from the category: Health and Medicine
This subject area encompasses research and studies in the field of human medicine.
Among the wide-ranging list of topics covered here are anesthesiology, anatomy, surgery, human genetics, hygiene and environmental medicine, internal medicine, neurology, pharmacology, physiology, urology and dental medicine.
Newest articles

Properties of new materials for microchips
… can now be measured well. Reseachers of Delft University of Technology demonstrated measuring performance properties of ultrathin silicon membranes. Making ever smaller and more powerful chips requires new ultrathin…

Floating solar’s potential
… to support sustainable development by addressing climate, water, and energy goals holistically. A new study published this week in Nature Energy raises the potential for floating solar photovoltaics (FPV)…

Skyrmions move at record speeds
… a step towards the computing of the future. An international research team led by scientists from the CNRS1 has discovered that the magnetic nanobubbles2 known as skyrmions can be…





















