
Reddy Lab, Johns Hopkins Medicine
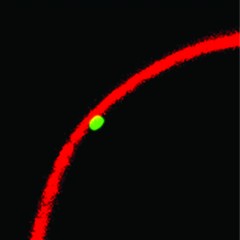
In mouse cells, the YY1 protein binds to a segment of DNA (green), leading it to attach to the lamina (red) at the edge of the nucleus.
Fast Facts:
• Directly inside the membrane of the cell’s nucleus is a meshwork of proteins called the lamina.
• Genes are turned off in large stretches of DNA that attach to the lamina.
• New work reveals what is needed to get DNA to stick, shedding light on how the body regulates its genes.
For a skin cell to do its job, it must turn on a completely different set of genes than a liver cell — and keep genes it doesn’t need switched off. One way of turning off large groups of genes at once is to send them to “time-out” at the edge of the nucleus, where they are kept quiet. New research from Johns Hopkins sheds light on how DNA gets sent to the nucleus’ far edge, a process critical to controlling genes and determining cell fate.
A report on the work appeared in the Jan. 5 issue of the Journal of Cell Biology.
“We discovered a DNA sequence and a specific set of protein tags that send DNA to the edge of the nucleus, where its genes get turned off,” says Karen Reddy, Ph.D., an assistant professor of biological chemistry at the Johns Hopkins University School of Medicine.
Picture the nucleus as a round room filled with double strands of DNA hanging in suspension as they are opened, closed, clipped, patched and read by proteins that come and go. At the edge of the nucleus, just inside its flexible walls, the lamina meshwork provides shape and support. But accumulating evidence from the past few years suggests that this meshwork is not just a structure, but is crucial to the cell’s ability to turn large segments of genes off in one fell swoop. It’s as though certain stretches of DNA feel a magnetic pull that keeps them clinging to the lamina in a state of “time-out,” inaccessible to the proteins that could be working on them.
This method of turning off entire segments of the genome is particularly useful during development, when each cell in the embryo takes on a different fate by making a different set of proteins, even though each contains the same set of genes. What was unknown is what marks a particular DNA segment to be sent to the lamina for some “quiet time.”
Reddy and her team began answering that question by comparing immature, embryonic, skinlike cells to mature immune system cells from mice. When they compared the segments of DNA clinging to the lamina in the two cell types, they found that differences occurred near genes that are used differently between the two. Additionally, the DNA regions that cling to the lamina were very consistent; there were no “grey areas” that were only sometimes associated with the lamina.
Next, the researchers chopped up the lamina-associated DNA segments and inserted individual pieces into the chromosomes of test cells, watching for the nearby chromosome segments to move to the lamina. They found that these segments were able to bind the protein YY1, and that YY1, when bound to a segment of DNA, was able to send the surrounding DNA to the lamina.
Reddy’s team also discovered two molecular tags that are needed for DNA to move to the lamina. The tags are found on the histone proteins that DNA coils around and are a classic form of “epigenetic regulation” — gene regulation that does not involve DNA sequence changes. It seems likely that YY1 is involved in summoning the proteins that attach the molecular tags to the histones. But whether YY1 has additional roles, like acting as a magnet to bring the DNA to the lamina, is unclear.
“This is the first time a specific combination of epigenetic modifications has been implicated in tethering DNA to the lamina,” says Reddy. “Now we have a lot of interesting questions to answer about how different types of cells use this mechanism to regulate different sets of genes.”
Other authors of the report include Jennifer Harr, Teresa Romeo Luperchio, Xianrong Wong, Erez Cohen and Sarah Wheelan of the Johns Hopkins University School of Medicine.
This work was supported by a grant from the National Institute of General Medical Sciences (1 R01 GM106024-01).
Contact Information
Catherine Kolf
Senior Communications Specialist
ckolf@jhmi.edu
Phone: 443-287-2251
Mobile: 443-440-1929












