Weeds in the brain
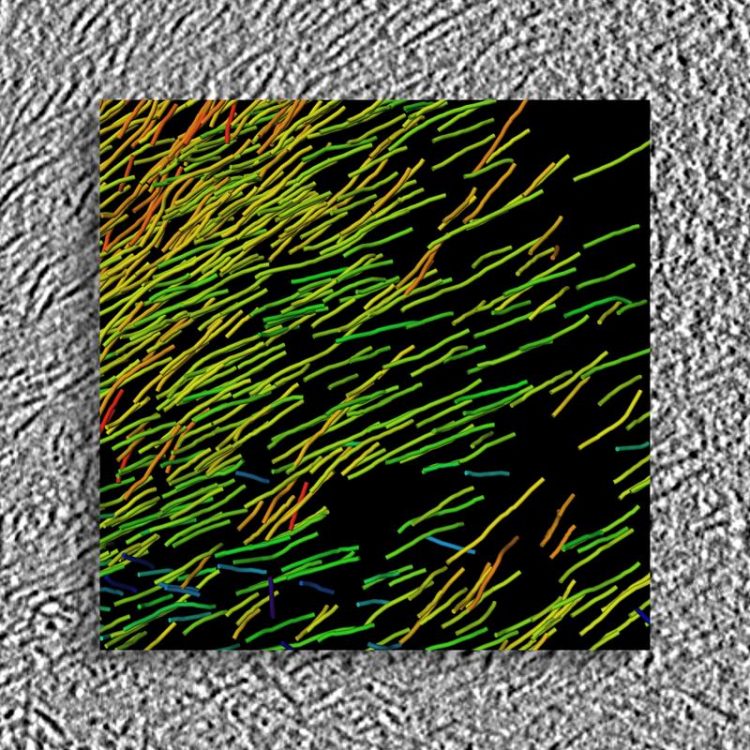
High-resolution 3D structure of huntingtin aggregates generated from cryo electon tomograms. Background: Raw data; Foreground: 3D Visualisation © MPI für Biochemie
Rampant weed growth – the nightmare of every hobby gardener. Trimming, cropping, cutting. Thorough garden maintenance is required. If this maintenance is neglected, weeds gain the upper hand and suppress the growth of crop and ornamental plants.
The same applies to proteins in our bodies: molecular machines, large protein complexes that control vital cellular processes, assume the responsibility of a gardener. These molecular machines ensure that proteins reach their correct conformations and tend to and care for them for the duration of their lifespans.
A matter of the correct form
In order to carry out its function, a protein needs to adopt its correct three-dimensional structure. The building blocks of proteins, the amino acids, are assembled into long chains and folded into a complex form. If the resulting structure is faulty, the defective proteins are broken down in a strictly regulated process.
If this does not occur properly, the misfolded proteins may aggregate forming clumps and deposits. Insoluble protein aggregates are toxic for cells. In the brain of patients suffering from neurodegenerative diseases such as Alzheimer’s, Parkinson’s, or Huntington’s, protein aggregates are often found.
If and how exactly these aggregates exert their toxic effects has not yet been explained. This is the question studied by the ToPAG (Toxic Protein AGgregation in neurodegeneration) consortium. A team of researchers in the departments of Wolfgang Baumeister, Ulrich Hartl and Rüdiger Klein has succeeded in decoding a 3D structure of the protein aggregates linked to Huntington’s disease within their intact cellular environment.
Microscopy, ice-cold
The breakthrough was enabled by a novel technique in structural research, cryo-electron tomography. In this technique, cells are flash-frozen and then, using an electron microscope, two-dimensional pictures are generated from different angles. The researchers can then assemble the generated pictures on a computer like the pieces of a 3D puzzle to generate a high-resolution model.
“With this method, we can take a snapshot of protein structures within intact cells, and determine with which additional cellular structures these proteins interact”, is how Rubén Fernández-Busnadiego, coordinator of the study, explains the special features of this technique.
When the scientists examined nerve cells with protein deposits under the microscope, they discovered inclusion bodies consisting of sticky, filamentous bundles of the huntingtin protein, so-called fibrils. In Huntington’s patients, a mutation in a single gene leads to defects in the huntingtin protein: The DNA, the blueprint for proteins, encoding huntingtin in these patients contains an abnormally high number of repeat copies of a particular sequence. As a result, the produced protein contains at its end multiple copies of a protein building block glutamine. This makes the faulty huntingtin proteins particularly sticky, and they easily clump into insoluble aggregates.
“Over time, more and more of these proteins become incorporated into aggregates”, explains Felix Bäuerlein, first author of the study. Staying with the gardening analogy: In brain cells, the aggregates proliferate like weeds. Where they have once spread and aren’t removed properly, the weeds multiply. And in the same way that these weeds spoil neatly tended flower beds and suppress the growth of other plants, so do the aggregated proteins interfere with the functioning of neighboring cellular components.
“If these protein deposits spread, they severely deform the membranes of cellular structures with which they come into contact. In some instances, this may lead to the tearing of the membrane”, says Bäuerlein. One organelle which is affected is the endoplasmic reticulum. In this way, the functioning of healthy organelles and proteins might be compromised. “We hypothesize that, little by little, the infrastructure of the cell is destroyed”, concludes Fernández-Busnadiego.
Previous therapies have been targeted only at the symptoms of neurodegenerative diseases, and there is no cure for patients with these conditions. “This insight into the structure of protein aggregates should improve our understanding of how aggregates exert their toxic effects on nerve cells. Our results open up an interesting perspective for further research into novel therapeutic approaches”, says Fernández-Busnadiego optimistically. [SiM]
Original publication:
F. Bäuerlein, I. Saha, A. Mishra, M. Kalemanov, A. Martínez-Sánchez, R. Klein, I. Dudanova, M.S. Hipp, F.U. Hartl, W. Baumeister, & R. Fernández-Busnadiego: In Situ Architecture and Cellular Interactions of PolyQ Inclusions, Cell, September 2017
DOI: 10.1016/j.cell.2017.08.009
—
Rubén Fernández-Busnadiego
Rubén Fernández-Busnadiego studied physics at the Universidad Complutense de Madrid in Spain. In 2010, he earned a PhD in Chemistry at the Technical University of Munich. Fernández-Busnadiego spent two years as a postdoctoral fellow at Yale University School of Medicine in New Haven, CT, USA. Since 2013, he is project group leader in the department Molecular Structural Biology of Wolfgang Baumeister. Fernández-Busnadiego and his team investigate the structural basis of toxic protein aggregation in neurodegenerative diseases at unprecedented resolution using novel microscopy techniques. For his work, he was awarded the FEBS Anniversary Prize in 2017.
The Max Planck Institute of Biochemistry
The Max Planck Institute of Biochemistry (MPIB) belongs to the Max Planck Society, an independent, non-profit research organization dedicated to top level basic research. As one of the largest Institutes of the Max Planck Society, 850 employees from 45 nations work here in the field of life sciences. In currently eight departments and about 25 research groups, the scientists contribute to the newest findings in the areas of biochemistry, cell biology, structural biology, biophysics and molecular science. The MPIB in Munich-Martinsried is part of the local life-science-campus where two Max Planck Institutes, a Helmholtz Center, the Gene-Center, several bio-medical faculties of two Munich universities and several biotech-companies are located in close proximity. (http://biochem.mpg.de)
ToPAG
Neurodegenerative diseases such as Alzheimer’s, Parkinson’s, and Huntington’s are characterized by toxic protein deposits in particular regions of the brain. How exactly these aggregates damage nerve cells and lead to their demise is the question which is researched by the ToPAG (Toxic Protein AGgregation in neurodegeneration) consortium, an association of scientists from the Max Planck Institutes in Martinsried outside Munich. This interdisciplinary research project is led by the departments of Wolfgang Baumeister, Ulrich Hartl and Matthias Mann at the MPI of Biochemistry and Rüdiger Klein at the MPI of Neurobiology. They employ a range of different methods of cellular biochemistry, proteomics, and cryo-electron tomography to decipher the mechanisms underlying the toxicity of protein aggregates. ToPAG is supported by the European Research Council (ERC). (http://www.topag.mpg.de)
Contact:
Dr. Rubén Fernández-Busnadiego
Molecular Structural Biology
Max-Planck-Institut für Biochemie
Am Klopferspitz 18
82152 Martinsried
E-Mail: ruben@biochem.mpg.de
www.biochem.mpg.de/baumeister
Dr. Christiane Menzfeld
Public Relations
Max-Planck-Institut für Biochemie
Am Klopferspitz 18
82152 Martinsried
Tel. +49 89 8578-2824
E-Mail: pr@biochem.mpg.de
www.biochem.mpg.de
http://www.biochem.mpg.de/en/news – More press releases of the MPI of Biochemistry
http://www.biochem.mpg.de – Website of the Institute
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

“Nanostitches” enable lighter and tougher composite materials
In research that may lead to next-generation airplanes and spacecraft, MIT engineers used carbon nanotubes to prevent cracking in multilayered composites. To save on fuel and reduce aircraft emissions, engineers…

Trash to treasure
Researchers turn metal waste into catalyst for hydrogen. Scientists have found a way to transform metal waste into a highly efficient catalyst to make hydrogen from water, a discovery that…

Real-time detection of infectious disease viruses
… by searching for molecular fingerprinting. A research team consisting of Professor Kyoung-Duck Park and Taeyoung Moon and Huitae Joo, PhD candidates, from the Department of Physics at Pohang University…





















