
Image by Josephine Leung, courtesy of the Stout lab, The Scripps Research Institute.
The new study provides insight into how a critical mitochondrial enzyme, transhydrogenase (TH), works in a process that is key to maintaining healthy cells.
The finding, published in the January 9 issue of Science, concerns nicotinamide nucleotide transhydrogenase (TH), an ancient evolutionary enzyme found throughout the animal kingdom as well as in plants and many simpler species. The enzyme is part of a process key to maintaining healthy cells and has also recently been linked to diseases such as diabetes and cancer.
“Despite its importance, TH has been one of the least-studied of mitochondrial enzymes,” said TSRI Associate Professor C. David Stout. “Our new study helps clear up some mysteries—suggesting how the enzyme structure might harness protons and indicating that its two sides are able to alternate functions, always staying in balance.”
Powering the Cell
In humans and other higher organisms, TH enzymes work within mitochondria, the tiny, double-hulled oxygen reactors that help power most cellular processes.
As a mitochondrion burns oxygen, it pumps protons (hydrogen atoms denuded of their electrons) out of its inner compartment (“matrix”), creating an excess of these charged particles just outside its inner membrane. TH enzymes, which are fixed at one end within this membrane, allow a one-by-one flow of protons back through the membrane within the matrix. This process—which is similar to that which makes ATP, the cell’s universal source of energy—has also been linked to the production of a compound called NADPH, which is crucial for defusing oxygen free radicals to maintain cell health.
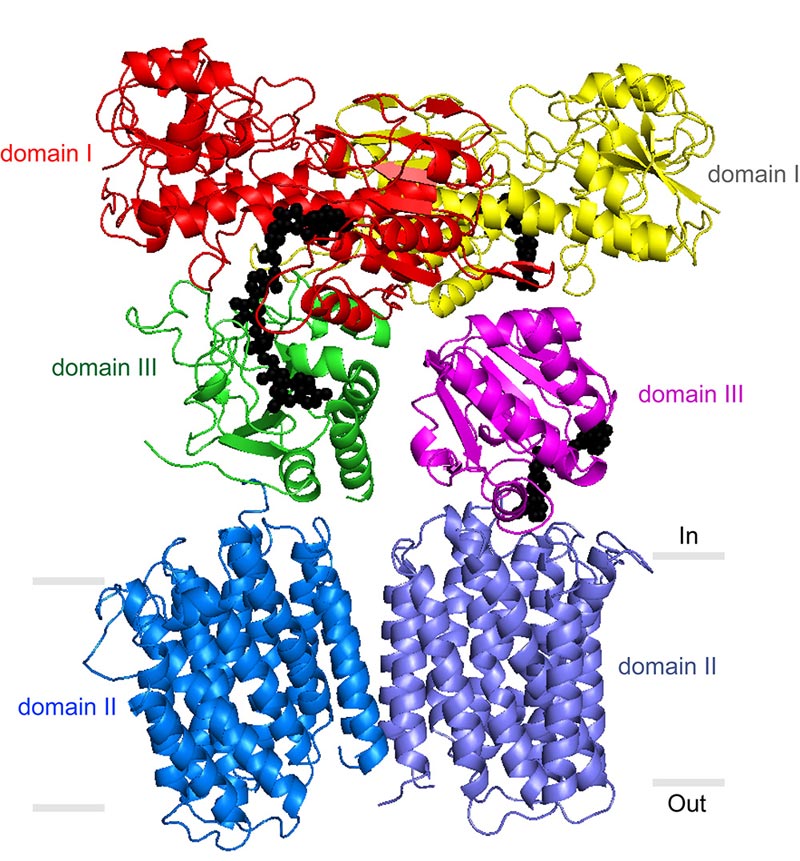
Stout’s laboratory and others have previously described portions of the TH enzyme that protrude from the membrane into the mitochondrial matrix. But a precise understanding of TH’s mechanism has been elusive. In its entirety, the enzyme has an exceptionally loose structure that makes it hard to evaluate using X-ray crystallography, the standard tool for determining the structures of large proteins at atomic-level resolution.
“Key details we’ve been lacking include the structure of TH’s transmembrane portion, and the way in which the parts assemble into the whole enzyme,” said Josephine H. Leung, a graduate student in the Stout laboratory who was lead author of the study.
New Clues to a Dynamic Structure
In the new study, thanks to technology developed by Professor Vadim Cherezov, now of University of Southern California, Leung and her colleagues were able for the first time to form crystals (neatly lined-up groupings) of the TH transmembrane portion and use X-ray crystallography to determine its structure—to an atomic-level resolution of 2.8 angstroms (280 trillionths of a meter).
The team also was able to grow crystals of the whole TH enzyme. These yielded a much lower-resolution structural image, but the researchers were able to enhance the resolution to 6.9 angstroms by plugging in data from crystallography of individual TH portions. In a further study, Professor Bridget Carragher and colleagues at the TSRI-based National Resource for Automated Molecular Microscopy (NRAMM) imaged individual copies of the enzyme to 18 angstroms using electron microscopy. Stout emphasized that such seamless collaborations at TSRI made this work possible: “Only an environment as at Scripps would enable the study of transhydrogenase.”
The electron microscopy data confirmed that TH naturally exists as a “dimer”—two identical copies bound together—and provided major clues to how TH manages to work in this conformation.
Directly above TH’s transmembrane structure, just inside the mitochondrial matrix, is the “domain III” structure that binds NADPH’s precursor molecule, NADP+, during conversion to NADPH. Structural biologists haven’t understood how two such structures could work side by side in the TH dimer and not interfere with each other’s activity. The new structural data suggest that these side-by-side structures are highly flexible and always have different orientations.
“Our most striking finding was that the two domain III structures are not symmetric—one of them faces up while the other faces down,” said Leung.
In particular, one of structures is oriented apparently to catalyze the production of NADPH, while the other is turned towards the membrane, perhaps to facilitate transit of a proton. The new structural model suggests that with each proton transit, the two domain III structures flip and switch their functions. “We suspect that the passage of the proton is what somehow causes this flipping of the domain III structures,” said Leung.
But much work remains to be done to determine TH’s precise structure and mechanism. For example, the new structural data provide evidence of a likely proton channel in the TH transmembrane region, but show only a closed conformation of that structure. “We suspect that this channel can have another, open conformation that lets the proton pass through, so that’s one of the details we want to study further,” said Leung.
“There are many experiments to follow,” Stout said.
Other co-authors of the study, “Division of labor in transhydrogenase by alternating proton translocation and hydride transfer,” were Robert B. Gennis, professor of biochemistry and biophysics at the University of Illinois at Urbana-Champaign, and a research associate in his laboratory, Lici A. Schurig-Briccio, who produced whole TH proteins for analysis and characterized the activity of TH when mutated at key structural sites; Jeffrey A. Speir of NRAAM; former NRAAM member Arne Moeller, now at Aarhus University; and Mutsuo Yamaguchi, staff scientist in the Stout laboratory at TSRI.
Support for the study was provided by the National Institutes of Health (5R01GM061545) and by the National Institute of General Medical Sciences (1R01GM103838, GM095600, GM073197 and P41GM103310).
About the Scripps Research Institute
The Scripps Research Institute (TSRI) is one of the world's largest independent, not-for-profit organizations focusing on research in the biomedical sciences. TSRI is internationally recognized for its contributions to science and health, including its role in laying the foundation for new treatments for cancer, rheumatoid arthritis, hemophilia, and other diseases. An institution that evolved from the Scripps Metabolic Clinic founded by philanthropist Ellen Browning Scripps in 1924, the institute now employs about 3,000 people on its campuses in La Jolla, CA, and Jupiter, FL, where its renowned scientists—including three Nobel laureates—work toward their next discoveries. The institute's graduate program, which awards PhD degrees in biology and chemistry, ranks among the top ten of its kind in the nation. For more information, see www.scripps.edu












