
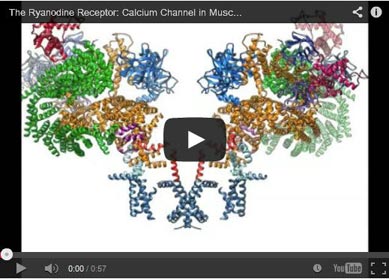
Whenever muscles contract, so-called ryanodine receptors come into play. Calcium ions, which are ultimately responsible for the contraction of muscle cells, are released from storage organs and flow through these ion channels. Defective ryanodine receptors can lead, for example, to cardiac arrhythmias or sudden heart failure. Resear
chers at the Max Planck Institute of Molecular Physiology in Dortmund have now analysed the three-dimensional structure of the ryanodine receptor. The researchers inserted the receptors into tiny nano-membranes in order to study the proteins in a milieu similar to their natural environment in cells.
With the help of electron cryo-microscopy and a new technique for detecting electrons, they were able to elucidate the structure of the receptor with high accuracy.
The animation shows how the protein changes its structure when calcium ions bind to it. Armed with this knowledge, scientists may be able to develop new materials in future to repair malfunctioning ryanodine receptors.
The ryanodine receptor forms a channel that is permeable for calcium ions. The animation illustrates how the protein changes its structure upon the binding of calcium.
Four helical domains in the center form an ion gate, which allows solely calcium to pass. The so-called EF-hand is the sensor that recognizes the ions. The domain is also known from other calcium-sensing proteins. It consists of charged amino acids and opens the gate region when calcium is bound.
Contact
Dr. Stefan Raunser
Max Planck Institute of Molecular Physiology, Dortmund
Phone: +49 231 133-2356
Fax: +49 231 133-2399
Email: stefan.raunser@mpi-dortmund.mpg.de
Dr. Peter Herter
Press and Public Relations
Max Planck Institute of Molecular Physiology, Dortmund
Phone: +49 231 133-2500
Email: peter.herter@mpi-dortmund.mpg.de
Original publication
Julian von der Ecken, Mirco Müller, William Lehman, Dietmar J. Manstein, Pawel A. Penczek & Stefan Raunser
Structure of the F-actin-tropomyosin complex
Nature, 1. December 2014, published online












