
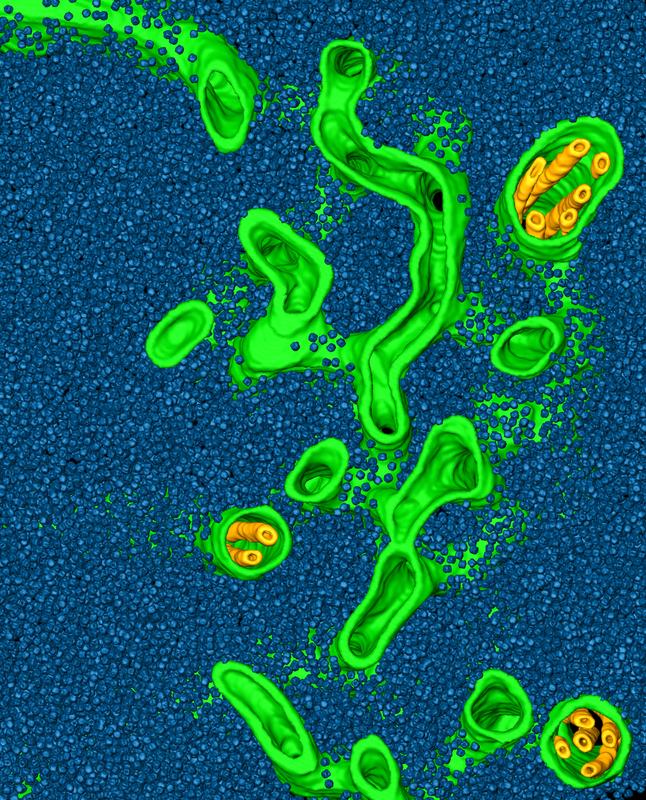
This image depicts a rendering of a cryo-electron tomogram of a Chlamydomonas pyrenoid, with tubule membranes (green and yellow) awash in a “sea” of Rubisco enzymes (blue).
© ScienceDirect
A warming planet
Our planet’s climate is changing. Each year brings record high temperatures that cause extreme weather, melting polar ice and rising ocean levels. Global warming is intensified by greenhouse gasses such as carbon dioxide, which prevent the escape of heat from our atmosphere.
Using energy from the sun in a process called photosynthesis, plants and algae act as natural air filters, removing carbon dioxide from the atmosphere while replacing it with the oxygen that we breathe. About half of the photosynthesis on Earth is performed in the ocean by single-celled algae. Many of these algae fix carbon dioxide more efficiently than land plants by concentrating most of their Rubisco into a microcompartment called a pyrenoid. Despite the importance of the pyrenoid to the global environment, until recently, it was unknown how this microcompartment assembles.
Visualizing every Rubisco within the pyrenoid
The first breakthrough in understanding pyrenoid assembly came when the team of Martin Jonikas, leader of the Carnegie/Stanford and Princeton groups, identified a linker protein in the green alga Chlamydomonas that binds Rubisco enzymes together within the pyrenoid. Without this “molecular glue,” the pyrenoid does not form. However, it was not known how the Rubisco proteins are organized within the pyrenoid, with classical electron microscopy studies suggesting that the pyrenoid is a highly ordered solid crystal.
To answer this question, the team lead by Benjamin Engel at the Max Planck Institute of Biochemistry used cryo-electron tomography to examine the molecular organization of the pyrenoid within Chlamydomonas cells that were frozen in their native state, avoiding the artefacts caused by sample preparation for classical electron microscopy.
This high-resolution imaging technique enabled Engel and colleagues to precisely measure the positions of the thousands of Rubisco enzymes within the pyrenoid. Instead of crystalline organization, they found that the pyrenoid only has short-range order. Engel explains this result: “If you compare our measurements to the organization of molecules inside liquids, there are very clear similarities. This suggests that pyrenoids are actually liquid-like structures.”
Like oil and water
In order to prove that the pyrenoid behaves like a liquid, Elizabeth Freeman Rosenzweig, first author of the study, used fluorescence microscopy to measure Rubisco movement within living cells. She used a high-powered laser to destroy the signal from fluorescent labels attached to Rubisco in half of the pyrenoid, while leaving the signal in the other half of the pyrenoid intact. Within minutes, the fluorescence spread throughout the pyrenoid, showing that the enzymes move around as they would in a liquid.
Thus, the pyrenoid is a liquid microcompartment floating within a second larger liquid compartment, the chloroplast. This is an example of “phase separation,” a physical phenomenon that has recently been shown to play a role in compartmentalizing many of the cell’s proteins. Freeman Rosenzweig uses an analogy to explain how it works: “Although the forces that cause the pyrenoid’s phase separation are different, it is easy to think about it terms of a familiar image: a dish of oil and vinegar that you might get at an Italian restaurant. The oil and vinegar are both liquids, but they don’t mix. The vinegar instead forms droplets that float in the pool of oil. Similarly, we think the pyrenoid forms a droplet within the liquid environment of the chloroplast.”
Freeman Rosenzweig also discovered that there is a special time when the “oil” of the chloroplast stroma and the “vinegar” of the pyrenoid do mix. As the single-celled algae divide into two daughter cells, the pyrenoid undergoes a “phase transition,” partially dissolving into the surrounding stroma of the chloroplast. Ordinarily, the remaining pyrenoid is pinched into two, with each daughter cell receiving half. However, sometimes this division fails, leaving one of the daughter cells with no pyrenoid.
The researchers observed that cells that failed to receive half of the pyrenoid could still form one spontaneously, or “de novo.” They suspect that each daughter cell receives some of the dissolved pyrenoid components, and that these components can condense into a new pyrenoid the way that raindrops condense from water vapor. “We think the pyrenoid dissolution before cell division and condensation after division may be a redundant mechanism to ensure that both daughter cells get pyrenoids,” Jonikas said. “That way, both cells will have this key organelle that's critical for assimilating carbon.”
Better crops for a changing world
Jonikas and his team have big plans for the applications of this knowledge. They want to engineer pyrenoids into crops such as wheat and rice to address problems including climate change and world hunger. “Understanding how algae can concentrate carbon dioxide is a key step toward the goal of improving photosynthesis in other plants,” Jonikas said.
“If we could engineer other crops to concentrate carbon, we could address the growing world demand for food.” The Jonikas group has even created their own mascot, Sammy the Chlamy, who uses a music video to teach us about the power of the pyrenoid: https://www.youtube.com/watch?v=B2ftWvnBanY
Some portions of this article were provided courtesy of the Princeton University Office of Communications. The music video was created by Jonathan Mann.
[SiM]
Original publication
E.S. Freeman Rosenzweig, B. Xu, L. Kuhn Cuellar, A. Martinez-Sanchez, M. Schaffer, M. Strauss, H.N. Cartwright, P. Ronceray, J.M. Plitzko, F. Förster, N.S. Wingreen, B.D. Engel, L.C.M. Mackinder & M.C. Jonikas. “The Eukaryotic CO2-Concentrating Organelle is Liquid-Like and Exhibits Dynamic Reorganization”. Cell, September 2017
DOI: 10.1016/j.cell.2017.08.008
About Benjamin Engel
Benjamin Engel’s work focuses on characterizing the molecular architecture of organelles, including the chloroplast. Using cryo-electron tomography, he and his team are able to visualize macromolecular complexes within the native cellular environment with high spatial resolution. Engel completed his undergraduate studies in Molecular and Cell Biology at the University of California, Berkeley, in the United States. In 2011, he received his Ph.D. from the University of California, San Francisco. Since then, he has worked as a postdoctoral fellow in the “Molecular Structural Biology” department of Wolfgang Baumeister at the Max Planck Institute for Biochemistry in Martinsried near Munich. He was awarded the Humboldt Postdoctoral Research Fellowship and MPIB Junior Research Award.
About the Max Planck Institute of Biochemistry
The Max Planck Institute of Biochemistry (MPIB) belongs to the Max Planck Society, an independent, non-profit research organization dedicated to top level basic research. As one of the largest Institutes of the Max Planck Society, 850 employees from 45 nations work here in the field of life sciences. In currently eight departments and about 25 research groups, the scientists contribute to the newest findings in the areas of biochemistry, cell biology, structural biology, biophysics and molecular science. The MPIB in Munich-Martinsried is part of the local life-science-campus where two Max Planck Institutes, a Helmholtz Center, the Gene-Center, several bio-medical faculties of two Munich universities and several biotech-companies are located in close proximity. (http://biochem.mpg.de)
About Martin Jonikas
Martin Jonikas is an Assistant Professor at Princeton University. His laboratory aims to transform our understanding of photosynthetic eukaryotes by developing and applying cutting-edge tools. He studied aerospace engineering as an undergraduate at the Massachusetts Institute of Technology. He then received his Ph.D. in 2009 from the University of California, San Francisco working with Jonathan Weissman, Maya Schuldiner and Peter Walter on high-throughput genetics and protein folding in the endoplasmic reticulum. Jonikas did not do a postdoc and started his laboratory directly after obtaining his Ph.D., as a faculty member at the Carnegie Institution for Science and an Assistant Professor by courtesy at Stanford University. After seven years at Carnegie, he moved his laboratory to Princeton. He is the recipient of several awards, including a 2016 Howard Hughes Medical Institute-Simons Foundation Faculty Scholar Award, a 2015 NIH New Innovator Award and a 2010 Air Force Young Investigator Award.
Contact:
Dr. Benjamin Engel
Dept. of Molecular Structural Biology
Max Planck Institute of Biochemistry
Am Klopferspitz 18
82152 Martinsried
Germany
Tel. +49 89 8578-2653
E-Mail: engelben@biochem.mpg.de
www.biochem.mpg.de
Dr. Christiane Menzfeld
Public Relations
Max Planck Institute of Biochemistry
Am Klopferspitz 18
82152 Martinsried
Germany
Tel. +49 89 8578-2824
Mail: pr@biochem.mpg.de
www.biochem.mpg.de
http://www.biochem.mpg.de/en/ – Website of the Max Planck Institute of Biochemisty
http://www.biochem.mpg.de/en/rd/baumeister – Website of the research department „Molecular Structural Biology“ (Wolfgang Baumeister)












