
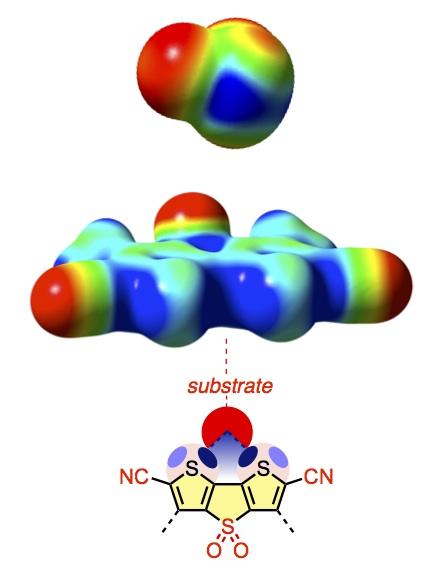
Highly electron-deficient, dark blue holes appear on the surface of sulfur atoms in the SF2 molecule, and on one of the best of the 'sulfurous' catalysts created by Professor Matile's group.
Credit: ©UNIGE
The active element in the molecule that initiates these transformations, known as the catalyst, is often hydrogen. However, a research team at the University of Geneva (UNIGE), Switzerland, has found that a sulfur atom, if carefully inserted into a molecule, can not only become an extremely effective catalyst but can also operate with greater precision. This discovery, published in Angewandte Chemie, has the potential to revolutionize the world of synthetic organic chemistry. It paves the way for the creation of new molecules that can be used in our daily life.
Creativity in fundamental research in chemistry consists of finding new ways to transform molecules and to build new molecular structures. To achieve this, the starting molecule needs to undergo a series of transformations until the molecular architecture of interest is achieved.
However, a molecule does not just change by itself – it has to be pushed by another molecule, the so-called catalyst. In nature, enzymes play this catalytic role. In chemistry and biology, the active element in catalysts is often the smallest possible atom – hydrogen.
“When we want to carry out a molecular transformation, we frequently use the hydrogen bond,” explains Stefan Matile, Professor in the Department of Organic Chemistry in the Faculty of Science at UNIGE, and director of the research project. “More precisely, we place the molecule that we want to transform, known as the substrate, in contact with hydrogen. The catalyst then attracts negative charge from the substrate, to the point where the molecule is so poor in negative charges that it is forced to seek contact with another substrate and, in order to maintain itself, to transform.” Hydrogen can be thought of as a vacuum cleaner that aspirates negative charges until the molecules are forced to come together and transform to compensate for the loss.
Sulfur increases precision
Professor Matile's team is interested in using bonds other than hydrogen bonds for catalysis and other activities. Most chemists consider these to be rather esoteric with little importance in the area of molecular transformation. However, when looking more closely at the sulfur atom in certain molecules, the UNIGE research team realized that the atom has a very localized area where it is extremely deficient in electrons, a sort of 'black hole'.
The team wanted to know whether this hole could act as a 'vacuum cleaner', like hydrogen, if it were placed in contact with a substrate. If this were the case, sulfur could be used as a catalyst, causing molecules to transform themselves. This somewhat unorthodox bond, known as a chalcogen bond, would thus replace the conventional hydrogen bond.
As Professor Matile further explains: “To test our hypothesis, we created and tested a series of molecular structures using chalcogen bonds of gradually increasing strength. We noticed that they not only work, but that they increase the speed of the transformation by more than a thousand times, as when there is no catalyst. Additionally, we achieved a degree of precision that is impossible with hydrogen bonds.” In fact, hydrogen's entire surface is 'electron poor'.
Thus, when it is playing the role of catalyst, the entire atom can come into contact with the substrate and suck up negative charges all over. However, with sulfur, only a small area can act as catalyst. This will enable chemists to be more precise in bringing the catalyst and substrate into contact, and thereby to exercise increased control over the transformation. This has the potential to revolutionize synthetic organic chemistry.
This discovery puts a new tool in the hands of chemists. It proves that it is now possible to use different approaches to carry out molecular transformations, and it opens up entirely new perspectives to the world of synthetic chemistry. Professor Matile's group will now attempt to build molecules that are not accessible with conventional hydrogen bonds. This opens the door for the creation of new materials.












