
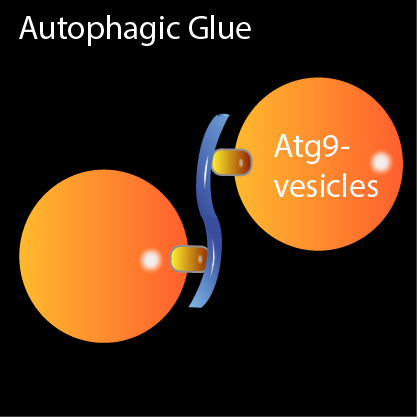
Two Atg9-vesicles (orange) are tethered by the Atg1-kinase complex (blue S-shaped structure). The vesicles are the raw material for a cellular trash bag.
Thomas Wollert © MPI of Biochemistry
Autophagy plays an important role in the cellular recycling process. It transports unwanted or damaged cytoplasmic material to the lysosomes, the cells’ recycling plants.
This is achieved by producing specialized waste bags, termed autophagosomes, that recognize the waste material, encase it, and transfer it to the recycling plant. Two protein components are essential for the production of these specialized waste bags. One of these is Atg9, a membrane protein embedded in small membrane vesicles, a kind of globule encased in a lipid membrane.
Atg9 vesicles serve as building blocks for the autophagosome waste bag. The second component, the Atg1 kinase complex, is a large protein complex consisting of five subunits. The scientists have now unraveled how both components are involved in the production of the autophagosome.
The scientists reproduced artificial Atg9 vesicle, the starting material for the waste bags, in a test tube. “By adding the Atg1 kinase complex we were able to show that one Atg1 kinase complex binds two Atg9 molecules, thus acting as a kind of clamp and connecting two Atg9 vesicles,” explains Yijian Rao, a member of the Molecular Membrane and Organelle Biology group headed by Thomas Wollert.
In the absence of waste two subunits of the Atg1 kinase complex can block the Atg9 binding site, thus inhibiting vesicle connections, which in turn prevents the formation of autophagosome waste bags. “This means the various subunits of the Atg1 kinase control membrane tethering and the production of the waste bag,” Rao further explains.
A small peptide that bears therapeutic potential is crucial for the medical application of the findings. The researchers were able to show that a certain peptide inhibits the Atg1 kinase complex in yeast cells. As Atg1 and Atg9 appear in both yeast cells and human cells the scientists assume that a similar compound can inhibit autophagy in human cells.
Cancer cells use autophagy in order to survive chemotherapy. Current cancer drugs induce damage in the cancer cells in order to kill them. The downside of the treatment is that such drugs not only attack cancer but also healthy cells.
One way to make cancer cells more vulnerable is to inactivate autophagy. “The inhibitor of the autophagic glue prevents the production of the waste bags and stops autophagy with high precision. This peptide could provide the basis for the development of a new anti-cancer drug or improve the efficiency of chemotherapeutic drugs currently in use,” Rao summarizes.
Original publication:
Rao, Y., Perna, M.B., Hofmann, B., Beier, V., Wollert, T.: The Atg1-kinase complex tethers Atg9-vesicles to initiate autophagy. Nature Communications, January 12, 2016
Doi: 10.1038/NCOMMS10338
Contact:
Dr. Thomas Wollert
Molecular Membrane and Organelle Biology
Max-Planck-Institut für Biochemie
Am Klopferspitz 18
82152 Martinsried
Germany
E-Mail: wollert@biochem.mpg.de
www.biochem.mpg.de/wollert
Dr. Christiane Menzfeld
Public Relations
Max Planck Institute of Biochemistry
Am Klopferspitz 18
82152 Martinsried
Germany
Tel. +49 89 8578-2824
E-Mail: pr@biochem.mpg.de
www.biochem.mpg.de
http://www.biochem.mpg.de – homepage max planck institute of biochemistry
http://www.biochem.mpg.de/wollert – homepage research group Thomas Wollert












