Soluble Elements from a New Corner of the Periodic Table
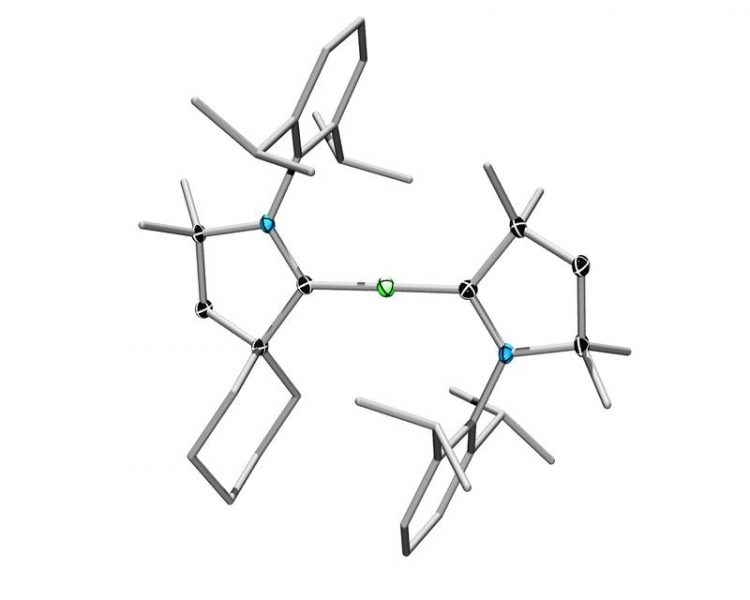
Beryllium in the center, flanked by two stabilizing cyclic ligands: another "world premiere" from Würzburg chemistry. (Graphic: Julia Schuster)
It is one of the more memorable experiments of high school chemistry lessons: when elemental sodium comes into contact with water it burns and explodes. Sodium simply isn't happy in its elemental form, making it highly reactive. This is more or less true for all of the other elements from the so-called “s-block” of the periodic table, to which sodium belongs.
A chemistry research group at the Julius-Maximilians-Universität (JMU) of Würzburg in Bavaria, Germany, has now, for the first time, tamed one of these “wild” s-block metals. The researchers constructed molecules that incorporate one atom of the alkaline earth metal beryllium in its elemental state. That the molecules do not immediately decompose at room temperature is thanks to stabilization by two cyclic ligands.
The breakthrough from the research team of Professor Holger Braunschweig is presented in the top-tier journal Nature Chemistry, thanks to the unexpectedly high stability of the molecules. These results from the JMU chemistry laboratories are expected to open a new era for the chemistry of the elements of this corner of the periodic table.
Promising candidates for challenging reactions
The incorporation of hydrogen and carbon monoxide into organic molecules is an example of one of the challenging chemical reactions carried out on huge scales in industry. Currently, these reactions are exclusively carried out with help from expensive heavy metals such as rhodium, palladium and platinum. For reasons of sustainability and cost, replacing these expensive catalysts with alternatives from the main group elements of the periodic table – many of which are abundant in the Earth's crust – would be a huge step forward.
This often means accessing the elemental states of these atoms in molecular systems. However, this is by no means trivial, as many of the potential candidate atoms ¬– sodium being an extreme example – are highly reactive in their elemental states. Recent success has been made with p-block elements such as silicon, tin and boron, while this new work is the first ever example with an s-block metal, beryllium.
Developing alternatives to toxic beryllium
“The only drawback of beryllium is its toxicity”, states Dr. Merle Arrowsmith, Alexander von Humboldt postdoctoral fellow in the group of Holger Braunschweig. Even more interesting would be to extend this chemistry to magnesium or calcium, elements that are both abundant and biocompatible, making them ideal as potential catalysts for important chemical reactions.
Given their success in incorporating elementary beryllium into a stable molecule, the chances are good that this could also work with other s-block metals. “Our discovery is a first step in capturing other s-block metal atoms in their elemental state, which we hope will promote reactions that usually only proceed with expensive heavy metals,” says Ph.D. student Julia Schuster, who synthesised the new molecules. The research group is currently developing similar methods for other s-block metals.
„Neutral zero-valent s-block complexes with strong multiple bonding“, Merle Arrowsmith, Holger Braunschweig, Mehmet Ali Celik, Theresa Dellermann, Rian D. Dewhurst, William C. Ewing, Kai Hammond, Thomas Kramer, Ivo Krummenacher, Jan Mies, Krzysztof Radacki, Julia K. Schuster. Nature Chemistry, DOI 10.1038/nchem.2542, Advance Online Publication 2016, June 6th
Contact
Prof. Dr. Holger Braunschweig, Institut für Anorganische Chemie, JMU, T (0931) 31-85260, h.braunschweig@uni-wuerzburg.de
http://www-anorganik.chemie.uni-wuerzburg.de/en/institute_of_inorganic_chemistry… Website of the Institute for Inorganic Chemistry
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Properties of new materials for microchips
… can now be measured well. Reseachers of Delft University of Technology demonstrated measuring performance properties of ultrathin silicon membranes. Making ever smaller and more powerful chips requires new ultrathin…

Floating solar’s potential
… to support sustainable development by addressing climate, water, and energy goals holistically. A new study published this week in Nature Energy raises the potential for floating solar photovoltaics (FPV)…

Skyrmions move at record speeds
… a step towards the computing of the future. An international research team led by scientists from the CNRS1 has discovered that the magnetic nanobubbles2 known as skyrmions can be…





















