
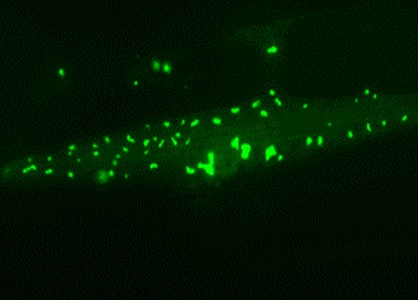
Muscle cell of a long-lived nematode worm: Chaperone-rich protein aggregates (green) accumulate and save the cell during aging.
Picture: Prasad Kasturi / Copyright: MPI of Biochemistry
Using a comprehensive approach, researchers in the teams of F.-Ulrich Hartl and Matthias Mann at the Max Planck Institute of Biochemistry in Martinsried near Munich now analyzed the changes in protein composition during aging.
The results published in the journal Cell show that the quantities of proteins undergo a severe shift. This also sheds new light on the origin and function of protein aggregates. The study also involves the groups of Michele Vendruscolo and Chris Dobson in Cambridge and of Richard Morimoto in Chicago.
Aging is a complex biological process which is accompanied by an increasing number of toxic protein aggregates in the cells. Scientists consider them the cause of various neurodegenerative disorders, such as Alzheimer’s, Huntington’s and Parkinson’s disease. However, their exact role remains poorly understood.
A collaborative team headed by F.-Ulrich Hartl at the MPIB now used the tiny nematode worm Caenorhabditis elegans (short: C. elegans) as a model organism to analyze the changes that occur in the proteome (the entirety of all proteins) during a lifespan. “The study is the most extensive of its kind in a whole organism quantifying more than 5,000 different proteins at multiple time points during aging”, explains Prasad Kasturi, equally contributing first author together with Dirk Walther.
The researchers were able to show that the proteome undergoes extensive changes as the worms age. About one third of the quantified proteins significantly change in abundance. The normal relation between different proteins, which is critical for proper cell function, is lost.
This shift overwhelms the machinery of protein quality control and impairs the functionality of the proteins. This is reflected in the widespread aggregation of surplus proteins ultimately contributing to the death of the animals.
Based on these findings, the researchers also analyzed how genetically changed worms with a substantially longer or shorter lifespan manage these changes. ”We found that proteome imbalance sets in earlier and is increased in short-lived worms. In contrast, long-lived worms coped much better and their proteome composition deviated less dramatically from that of young animals”, as Kasturi says.
Surprisingly, the long-lived worms increasingly deposited surplus and harmful proteins in insoluble aggregates, thus relieving pressure on the soluble, functional proteome. However, in contrast to the aggregates found in short-lived animals, these deposits were enriched with helper proteins – the so-called molecular chaperones – which apparently prevented the toxic effects normally exerted by aggregates.
“These findings demonstrate that the cells specifically accumulate chaperone-rich protein aggregates as a safety mechanism. Therefore, the aggregates seem to be an important part of healthy aging”, Kasturi explains. Indeed, it is known that insoluble protein aggregates also accumulate in the brains of healthy elderly people.
So far, researchers assumed that neurodegeneration and dementia appear to be mainly caused by aberrant protein species accumulating in aggregates. This assumption may now being tested again: “Clearly, aggregates are not always harmful. Finding ways to concentrate harmful proteins in insoluble deposits might be a useful strategy to avoid or postpone neurodegenerative diseases as we age”, F.-Ulrich Hartl classifies the results. [HS]
Original publication:
Walther DM*, Kasturi P*, Zheng M, Pinkert S, Vecchi G, Ciryam P, Morimoto RI, Dobson CM, Vendruscolo M, Mann M and Hartl FU: Widespread Proteome Remodeling and Aggregation in Aging C. elegans. Cell, May 7, 2015
DOI: 10.1016/j.cell.2015.03.032
Contact:
Prof. Dr. F.-Ulrich Hartl
Cellular Biochemistry
Max Planck Institute of Biochemistry
Am Klopferspitz 18
82152 Martinsried
Germany
E-Mail: uhartl@biochem.mpg.de
http://www.biochem.mpg.de/hartl
Anja Konschak
Public Relations
Max Planck Institute of Biochemistry
Am Klopferspitz 18
82152 Martinsried
Germany
Tel. +49 89 8578-2824
E-Mail: konschak@biochem.mpg.de
http://www.biochem.mpg.de
http://www.biochem.mpg.de/en/news/pressroom – More press releases of the MPI of Biochemistr
http://www.biochem.mpg.de/hartl – Website of the Research Department “Cellular Biochemistry” (F.-Ulrich Hartl)












