
Pictured together for the first time: A chemokine and its receptor
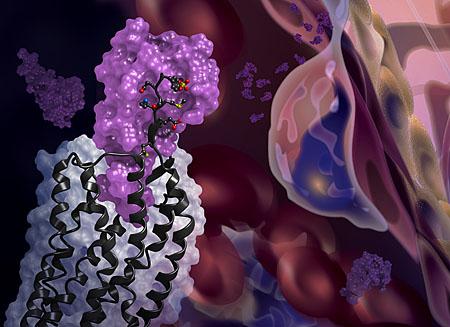
The newly solved structure of the CXCR4 receptor (black) in complex with a chemokine (purple surface). The background shows cell migration, a process driven by chemokines interacting with receptors on cell surfaces.
Credit: Katya Kadyshevskaya, USC
Researchers at University of California, San Diego Skaggs School of Pharmacy and Pharmaceutical Sciences and the Bridge Institute at the University of Southern California report the first crystal structure of the cellular receptor CXCR4 bound to an immune signaling protein called a chemokine. The structure, published Jan. 22 in Science, answers longstanding questions about a molecular interaction that plays an important role in human development, immune responses, cancer metastasis and HIV infections.
“This new information could ultimately aid the development of better small molecular inhibitors of CXCR4-chemokine interactions — inhibitors that have the potential to block cancer metastasis or viral infections,” said Tracy M. Handel, PhD, professor of pharmacology at UC San Diego and senior author of the study.
CXCR4 is a receptor that sits on the outer surface of cells, sticking out like an antenna. When it receives a message, in the form of signaling molecules called chemokines, the receptor binds the chemokines and transmits the message to the inside of the cell. This signal relay helps cells migrate normally during development and inflammation. But CXCR4 signaling can also play a role in abnormal cell migration, such as when cancer cells metastasize. CXCR4 is infamous for another reason: HIV uses it to bind and infect human immune cells.
Despite its far-reaching consequences, researchers have long lacked data to show how exactly the CXCR4-chemokine interaction occurs, or even how many CXCR4 receptors a single chemokine molecule might simultaneously engage. This is because membrane receptors like CXCR4 are exceptionally challenging structural targets. The difficulty dramatically increases when studying such receptors in complexes with the proteins they bind.
To overcome these experimental challenges, Handel's team used a novel approach. They combined computational modeling and a technique known as disulfide trapping to stabilize the complex. Once stabilized, the researchers were able to use X-ray crystallography to determine the CXCR4-chemokine complex's 3D atomic structure.
This is the first time that a receptor like CXCR4 has been crystallized with a protein binding partner and the results revealed several new insights. First, the new crystal structure shows that one chemokine binds to just one receptor. Additionally, the structure reveals that the contacts between the receptor and its binding partner are more extensive than previously thought — it is one very large contiguous surface of interaction rather than two separate binding sites.
“The plasticity of the CXCR4 receptor — its ability to bind many unrelated small molecules, peptides and proteins — is remarkable,” said Irina Kufareva, PhD, a computational scientist at UC San Diego and co-corresponding author of the study. “Our understanding of this plasticity may impact the design of therapeutics with better inhibition and safety profiles.”
“With more than 800 members, seven-transmembrane receptors like CXCR4 are the largest protein family in the human genome,” added Raymond Stevens, PhD, provost professor and director of the Bridge Institute at the University of Southern California and co-corresponding author. “Each new structure opens up so many doors to understanding different aspects of human biology, and this time it is about chemokine signaling.”
###
Study co-authors include Ling Qin, Lauren G. Holden, Yi Zheng, Chunxia Zhao and Ruben Abagyan, UC San Diego Skaggs School of Pharmacy; Chong Wang, Gustavo Fenalti, Huixian Wu, Gye Won Han, The Scripps Research Institute; and Vadim Cherezov, University of Southern California (previously at The Scripps Research Institute).
This research was made possible by the PSI:Biology program funded by the National Institute of General Medical Sciences at the National Institutes of Health (NIH). This research was also funded, in part, by NIH grants R01GM071872, U01GM094612, R01GM081763, R21AI101687, U54GM094618, Y1-CO-1020 and Y1-GM-1104, and the Pharmaceutical Research and Manufacturers of America Foundation.












