In “Cell”: Floppy but fast
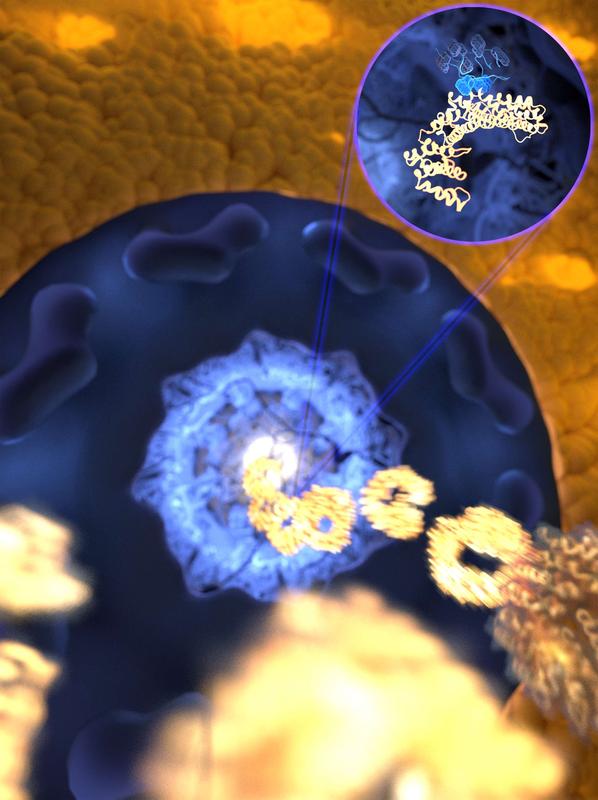
The ultrafast and yet selective binding allows the receptor(gold) to rapidly travel through the pore filled with disordered proteins (blue) into the nucleus, while any unwanted molecules are kept out Image: Mercadante /HITS
Proteins can recognize one another. Each engages very specifically with only a subset of the many different proteins present in the living cell, like a key slotting into a lock. But what if the key is completely flexible, as is the case for so-called intrinsically disordered proteins (IDPs)?
The research teams headed by Edward Lemke at the European Molecular Biology Laboratory (EMBL) in Heidelberg, Frauke Gräter at the Heidelberg Institute for Theoretical Studies (HITS) and Martin Blackledge at the Institut de Biologie Structurale, (IBS) in France, addressed this question in a highly interdisciplinary collaboration, combining molecular simulations, single molecule fluorescence resonance energy transfer (FRET), nuclear magnetic resonance (NMR), stopped flow spectroscopy and in-cell particle tracking.
Unexpectedly, they found that flexible, spaghetti-like proteins can be good – maybe even better than solid protein blocks – at being recognised by multiple partners. And they can do so very fast, while still retaining the high specificity the cell needs. In fact, this could be why these disordered molecules are more common in evolutionarily higher organisms, the researchers surmise.
Researchers had assumed that when an IDP ‘key’ needed to bind to its lock, it rearranged itself to become more rigid, but experiments in the Lemke lab hinted otherwise. “The pioneering single molecule experiments undertaken at EMBL showed for the particular interaction of a receptor with a disordered protein just nothing: the flexible protein stayed as flexible even when bound to its receptor” says Davide Mercadante (HITS).
This prompted him to study the very same interaction on the computer. The surprising result was that the high flexibility of the IDP actually helps it bind to its lock – in this case, a nuclear transport receptor, which shuttles proteins into the nucleus. The simulations even suggested the binding to be ultrafast – faster than any other association of that kind recorded to date.
“The computational data indicated that we might have identified a new ultrafast binding mechanism, but it took us three years to design experiments to prove the kinetics in the lab,” Iker Valle Aramburu (EMBL) recalls. “In the end, we had a remarkably perfect match.”
The results now help to understand a long-standing paradox: “For a cell to be viable, molecules must constantly move into and out of its nucleus”, says Edward Lemke (EMBL). ”Our findings explain the so-called transport paradox – that is, how this shuttling can be so very fast while remaining specific so that unwanted molecules cannot pass the barrier that protects our genome.”
The new study suggests that many binding motifs at the surface of the IDP create a highly reactive surface that together with the very high speed of locking and unlocking ensures efficient proof-reading while the receptors to travel so fast through a pore filled with other IDPs.
“This is likely a new paradigm for the recognition of intrinsically disordered proteins.” says Frauke Gräter (HITS). Since around 30-50% of the proteins in human cells are disordered, at least in some regions of the protein, the results may also provide a rationale for how recognition information can be processed very fast in general – which is vital to cells.
Other researchers involved in the study are working at the IBS Grenoble / France, and Cambridge University / UK.
Publication in “Cell”, Plasticity of an ultrafast interaction between nucleoporins and nuclear transport receptors
http://www.cell.com/cell/abstract/S0092-8674%2815%2901264-7
Sigrid Milles, Davide Mercadante, Iker Valle Aramburu, Malene Ringkjøbing Jensen, Niccolò Banterle, Christine Koehler, Swati Tyagi, Jane Clarke, Sarah L Shammas, Martin Blackledge, Frauke Gräter, Edward A Lemke
DOI: http://dx.doi.org/10.1016/j.cell.2015.09.047
Press contact:
Sonia Furtado Neves
EMBL Press Officer & Deputy Head of Communications
Tel.: +49 (0)6221 387 8263
Fax: +49 (0)6221 387 8525
sonia.furtado@embl.de
http://s.embl.org/press
Dr. Peter Saueressig
Head of Communications
Heidelberg Institute for Theoretical Studies (HITS)
Phone: +49-6221-533245
peter.saueressig@h-its.org
www.h-its.org
Twitter: @HITStudies
Scientific contact:
Dr. Frauke Gräter
Heidelberg Institute for Theoretical Studies (HITS)
Phone: +49-6221-533267
frauke.graeter@h-its.org
Dr. Edward Lemke
Structural and Computational Biology Unit, Cell Biology and Biophysics Unit, (EMBL)
Phone: +49-6221-387 8536
lemke@embl.de
http://www.h-its.org/en-presse/in-cell-floppy-but-fast/ HITS press release
http://www.cell.com/cell/abstract/S0092-8674%2815%2901264-7 Publication in “Cell”
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

High-energy-density aqueous battery based on halogen multi-electron transfer
Traditional non-aqueous lithium-ion batteries have a high energy density, but their safety is compromised due to the flammable organic electrolytes they utilize. Aqueous batteries use water as the solvent for…

First-ever combined heart pump and pig kidney transplant
…gives new hope to patient with terminal illness. Surgeons at NYU Langone Health performed the first-ever combined mechanical heart pump and gene-edited pig kidney transplant surgery in a 54-year-old woman…

Biophysics: Testing how well biomarkers work
LMU researchers have developed a method to determine how reliably target proteins can be labeled using super-resolution fluorescence microscopy. Modern microscopy techniques make it possible to examine the inner workings…





















