
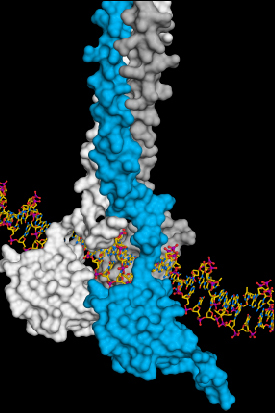
Three HSF1 molecules (white, blue, grey) associate to ensure stable interaction between HSF1 and DNA. This activates the production of cellular crisis helpers, the heat-shock proteins.
Tobias Neudegger © MPI of Biochemistry
When there is an accident or a house fire, we call the police or the fire services. A control room quickly coordinates emergency operations.
The cells in our bodies also have helpers in a crisis; the heat-shock proteins. These are triggered in response to cellular stress, such as high temperature, UV radiation or cancer. Heat-shock proteins help other proteins maintain their functional structure and eliminate denatured proteins to counter the abnormal cellular situation.
In cells, the operator in the control room is HSF1, heat-shock transcription factor 1. It binds certain DNA sequences that encode the “assembly instructions” for the cellular helpers. When HSF1 is activated, the production of functional heat-shock proteins is triggered.
Andreas Bracher and his team in Prof. Hartl’s Department of Cellular Biochemistry at the Max Planck Institute of Biochemistry in Martinsried have demonstrated exactly how HSF1 binds DNA.
“Using X-ray crystallography, we studied the exact molecular arrangement,” explains Tobias Neudegger, a member of Bracher’s team and first author of the study. Proteins consist of long strands of amino acids which adopt a certain three-dimensional structure in order to become functionally active.
“We were able to show how three identical HSF1 molecules associate in case of cellular stress. That is how a stable DNA-HSF1 interaction occurs. If HSF1 is not bound to DNA, each individual HSF1 molecule is stored in an inactive state in the cell,” Neudegger explains.
The increased production of heat-shock proteins could be advantageous for the treatment of diseases. “Now that we know the HSF1 structure, drugs can be developed to activate or deactivate HSF1 and thus stimulate or inhibit the production of cellular helpers,” says Bracher, describing potential future HSF1 research.
Incorrectly folded proteins in the cells could be repaired or denatured proteins more easily eliminated. Incorrectly folded proteins are usually found in connection with Huntington’s disease, Alzheimer’s and Parkinson’s disease, as well as in cancer cells.
Original publication:
T. Neudegger, J. Verghese, M. Hayer-Hartl, F. U. Hartl & A. Bracher: Structure of human heat-shock transcription factor 1 in complex with DNA. Nature Structural & Molecular Biology, February 2016
DOI: 10.1038/nsmb.3149
Dr. Andreas Bracher
Department of Cellular Biochemistry
Max Planck Institute of Biochemistry
Am Klopferspitz 18
82152 Martinsried
E-Mail: bracher@biochem.mpg.de
www.biochem.mpg.de/hartl
Dr. Christiane Menzfeld
Public Relations
Max Planck Institute of Biochemistry
Am Klopferspitz 18
82152 Martinsried
Germany
Tel. +49 89 8578-2824
E-Mail: pr@biochem.mpg.de
www.biochem.mpg.de
http://www.biochem.mpg.de/en/news – More press releases of the MPI of Biochemistry
http://www.biochem.mpg.de/hartl – Website of the Research Department “Cellular Biochemistry” (F.-Ulrich Hartl)












