
Understanding Toxic Protein Aggregates in Neurodegenerative Disorders
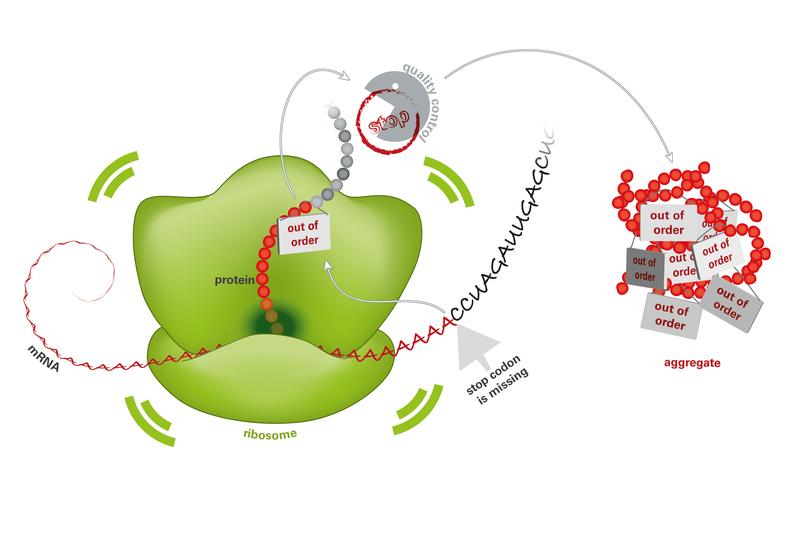
Mistakes in the blueprint for proteins (mRNA) lead to the production of useless proteins in the ribosomes. Since the quality control is broken, these proteins accumulate and form toxic aggregates.
Monika Krause © MPI of Biochemistry
In order to be able to treat neurodegenerative disorders in future, researcher Ulrich Hartl, Head of the Department of Cellular Biochemistry at the Max Planck Institute of Biochemistry, and his team have for many years been studying the cellular causes for the death of nerve cells. A determining cause is believed to be protein deposits – aggregates of misfolded proteins.
“We were able to show that the formation of aggregates is promoted by defects in the protein blueprint and these are not detected by the internal quality control machinery”, explains Young-Jun Choe, first author of the study together with Sae-Hun Park.
In each cell, proteins perform vital functions, acting as small molecular machines. “DNA can be envisaged as a huge library of protein blueprints that are located in the cell nucleus. To manufacture a protein, a copy of the blueprint, the mRNA, is first made. This is then directed from the cell nucleus to the ribosomes, which then build the protein from amino acids”, says Choe.
The mRNA contains a start signal, the information about the protein structure, a stop signal and, at the end, a poly(A) tail. If the blueprint is damaged, for example due to radiation or mutagenic substances, this can lead to the loss of this stop signal.
As a result, once the protein has been manufactured in the ribosomes, the completed protein cannot be released. Instead, the poly(A) tail is interpreted as the blueprint and additional amino acids are attached. The lysine chain that is positively charged as a result blocks the protein factory and the manufacture of protein comes to a standstill.
Healthy cells have a very efficient quality control process when it comes to the manufacture of protein. Misfolded and useless proteins are selected, repaired or rapidly destroyed. Ltn1p is an important component of quality control. “If Ltn1p is not active in pathologically modified cells or if other components of quality control are missing, defective proteins accumulate and form aggregates in cell interiors”, says Park.
Using a mouse model, the researchers can now demonstrate the fatal consequences of a quality control malfunction. Animals with the relevant mutation show symptoms of advanced neurodegeneration and a restricted ability to move.
The protein aggregates that develop have a sticky surface and act as a seed. They ultimately also bind functioning proteins, which are free of defects and vital for the cell. As a result, the cell is destabilized and, in the long run, is damaged. Interestingly, according to Ulrich Hartl, the cell seems to follow a known pattern in this regard.
“We already know from previous studies on the protein huntingtin, which spontaneously forms aggregates and is responsible for the development of the neurodegenerative disorder Huntington's disease, that protein aggregates also bind to essential proteins that have no defects.”
“Our results not only demonstrate a potential mechanism for the development of neurodegenera-tive disorders but we have also found another example of the way in which proteins can form aggregates and damage the cell. This confirms our assumption that the suppression of the aggregation of proteins represents a promising therapeutic approach for a large number of neurodegenerative disorders that are currently still incurable”, says Hartl, summarizing the results of the study.
Original publication:
Y.-J. Choe & S.-H. Park, T. Hassemer, R. Körner, L. Vincenz-Donnelly, M. Hayer-Hartl & F.-U. Hartl: Failure of RQC machinery causes protein aggregation and proteotoxic stress. Nature, February 2016
DOI: 10.1038/nature16973.
Contact:
Prof. Dr. F.-Ulrich Hartl
Department of Cellular Biochemistry
Max Planck Institute of Biochemistry
Am Klopferspitz 18
82152 Martinsried
E-Mail: uhartl@biochem.mpg.de
www.biochem.mpg.de/hartl
Dr. Christiane Menzfeld
Public Relations
Max Planck Institute of Biochemistry
Am Klopferspitz 18
82152 Martinsried
Germany
Tel. +49 89 8578-2824
E-Mail: pr@biochem.mpg.de
www.biochem.mpg.de
http://www.biochem.mpg.de/en/news – More press releases of the MPI of Biochemistry
http://www.biochem.mpg.de/hartl – Website of the Research Department “Cellular Biochemistry” (F.-Ulrich Hartl)












