How a Wound Closes
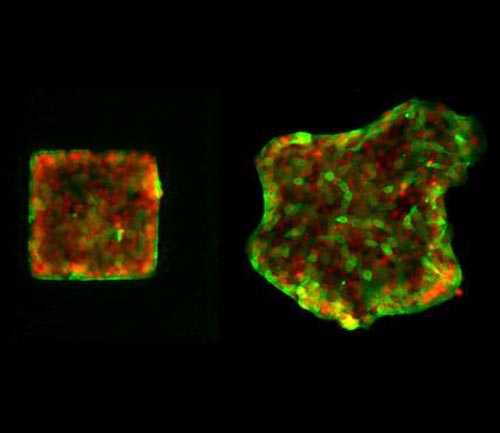
Epithelial cells move collectively out of their original shape (left) into the environment (right). Localisation of Merlin is shown in green, the cell nuclei in red. Picture: Max Planck Institute for Intelligent Systems
For wounds to close, cells need to move collectively in one direction in a coordinated fashion. Until now the central molecular mechanism that allows cells to coordinate these movements over larger distances has been unclear. Now researchers from Heidelberg University and the Max Planck Institute for Intelligent Systems in Stuttgart have succeeded in decoding it. Collective cell migration is not only important in wound healing, but also in the development of the embryo and even of cancer. The results of their research, published in the journal “Nature Cell Biology”, have tremendous implications for all three of these areas.
“The collective migration of cells and biological systems is one of the most important natural phenomena and occurs in nature at different levels and length scales. We have now identified the key molecular player and the related mechanism that controls the collective migration of epithelial cells, that is the covering layer of skin cells,” explains Prof. Dr. Joachim Spatz of the Institute for Physical Chemistry at Heidelberg University and the Max Planck Institute for Intelligent Systems. In their investigation, the researchers introduce a complete molecular mechanism that focuses on the protein called Merlin. The results link intercellular mechanical forces to collective cell movements and also demonstrate how local interactions give rise to collective dynamics at the multicellular level. “They create an analogy with what we already know about collective movements observable in both the biological and physical world,” explains Prof. Spatz.
The researcher compares the process of cell migration to running a marathon. “At the level of the organism, an individual in a collective consciously tries to align its movements with those of its neighbours, which involves orchestrated sensing and action.” Within a cellular collective, these two processes are linked via signal transduction pathways. There is a lead cell in the collective, similar to the leader in a marathon. It is mechanically connected to its follower cells by cell-to-cell contacts. The forward motion of the lead cell puts mechanical tension on the follower cells, according to Spatz. The merlin protein senses this mechanical tension and initiates spatially polarised following movement. This transmits the mechanical tension among the follower cells from one cell to the next. The follower cells respond by forming ‘leg-like’ protrusions directed at the lead cell in order to move forward.
“Until now it has been unclear what molecular link connects these two events, sensing and action,” says Joachim Spatz. “Our study now shows how the mechanosensitive Merlin protein converts cellular forces to collective cell motions by acting as a mechanochemical transducer. What’s truly astonishing is that Merlin is the only protein in the responsible signal network that conveys this property to cellular collectives – that there are no replacement mechanisms. If Merlin fails, the cells lose their ability to move collectively and trigger the related medically relevant, pathophysiological properties in the organism”.
The major player in the study, Merlin, is also a known tumour suppressor that is responsible for several types of cancer. Merlin is also a regulator of the Hippo pathway, an important signal pathway in biology that controls cell proliferation and organ size. It has been preserved in evolution since the emergence of primitive multicellular organisms. “It’s exciting to see a connection between these seemingly disparate fields, linked by a Merlin-mediated signalling mechanism,” says the researcher.
Researchers from the Hamamatsu Tissue Imaging and Analysis (TIGA) Center at the BioQuant Centre of Ruperto Carola and the National Center for Tumor Diseases (NCT) Heidelberg also participated in the study.
Original publication:
T. Das, K. Safferling, S. Rausch, N. Grabe, H. Boehm, J. Spatz: A molecular mechanotransduction pathway regulates collective migration of epithelial cells. Nature Cell Biology (published online 23 February 2015), doi: 10.1038/ncb3115
Contact:
Prof. Dr. Joachim Spatz
Institute of Physical Chemistry
Phone: +49 6221 54-4942
joachim.spatz@urz.uni-heidelberg.de
Communications and Marketing
Press Office
Phone: +49 6221 54-2311
presse@rektorat.uni-heidelberg.de
Media Contact
More Information:
http://www.uni-heidelberg.deAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Superradiant atoms could push the boundaries of how precisely time can be measured
Superradiant atoms can help us measure time more precisely than ever. In a new study, researchers from the University of Copenhagen present a new method for measuring the time interval,…

Ion thermoelectric conversion devices for near room temperature
The electrode sheet of the thermoelectric device consists of ionic hydrogel, which is sandwiched between the electrodes to form, and the Prussian blue on the electrode undergoes a redox reaction…

Zap Energy achieves 37-million-degree temperatures in a compact device
New publication reports record electron temperatures for a small-scale, sheared-flow-stabilized Z-pinch fusion device. In the nine decades since humans first produced fusion reactions, only a few fusion technologies have demonstrated…





















