Disabling infection-fighting immune response speeds up wound healing in diabetes
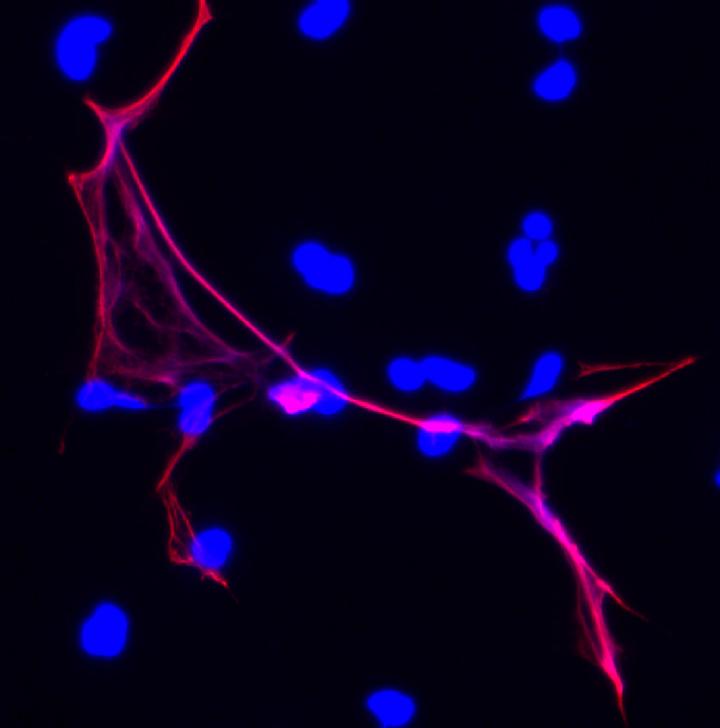
One of the body's tools for fighting off infection in a wound may actually slow down the healing process, according to new research published online in Nature Medicine on June 15, 2015, by a team that includes scientists at Penn State University, Harvard University, and Boston Children's Hospital. The researchers have speeded up wound healing in diabetic mice by preventing immune cells called neutrophils from producing structures called NETs (neutrophil extracellular traps) that trap and kill bacteria. In this image the structure colored in blue is chromatin -- the condensed form of DNA that the cell remodels to form chromosomes. The enzyme PAD4 decondenses chromatin by loosening up the interaction between DNA and special proteins called histones. The histones modified by PAD4 are shown in fuchsia. This process helps to form both a bacteria-killing NET -- which is comprised of infection-combatting white blood cells called neutrophils -- and the fluffy, scattered ball that comprises a blood clot. Credit: Wang lab, Penn State University. (Image originally was published in the Journal of Cell Biology).
“In the fight against bacterial infection, NETs cause collateral damage that slows healing,” said Yanming Wang, associate professor of biochemistry and molecular biology at Penn State and a member of the research team.
NETs are thought to reduce the risk of infection in a wound but they also form a dense, toxic mesh that interferes with the mobilization of new healthy cells and hinders tissue repair. The process is even more of a problem in individuals with diabetes, whose neutrophils produce more NETs. As a result, delayed wound healing is a common complication of both type 1 and type 2 diabetes.
To see how diabetes increases a neutrophil's ability to produce NETs, the researchers examined neutrophil cells from patients with either type 1 or type 2 diabetes. They found that these neutrophil cells contained four times the normal amount of the PAD4 enzyme — a protein that catalyzes the production of NETs. Further experiments revealed that neutrophils from healthy donors or mice that were exposed to excessive glucose — mimicking diabetes — also were more likely to release NETs than neutrophils that were exposed to normal glucose levels.
Diabetic mice in the study had more NETs in wounds and healed more slowly than normal mice. However, when the team examined diabetic mice that lacked the PAD4 enzyme they found that the wounds of these mice healed more quickly. “Neutrophils of individuals with diabetes are primed to form NETs by high levels of PAD4, but when we eliminate or control the expression of the PAD4 enzyme in mice with diabetes, we can prevent NETs from forming and speed up healing,” Wang said. “It remains to be tested if pharmacological intervention of PAD4 activity will benefit the healing process.”
“NETs predispose patients to inflammation, heart disease, and deep-vein thrombosis — dangerous blood clots that form within veins deep inside the body — all of which are elevated in patients with diabetes,” said the senior author of the study Denisa Wagner, senior investigator of the Program in Cellular and Molecular Medicine at Boston Children's Hospital and Edwin Cohn Professor of Pediatrics at Harvard Medical School. “Any injury that causes inflammation will result in the production of NETs, and we think that if the injury involves skin repair, NETs will hinder the repair process.”
When the skin is cut or broken, the body mobilizes a complicated array of cells and proteins to stop bleeding, prevent infection by triggering inflammation, and start the healing process. As part of the inflammatory response, neutrophils, which ingest and destroy bacteria, expel their own chromatin — a mix of DNA and associated proteins — in the form of NETs within the wound.
To see whether breaking up the NETs would have an effect similar to preventing their production, the research team treated mice with DNase 1 — an enzyme that breaks up DNA and therefore can destroy NETs. After three days, wounds on DNase 1-treated diabetic animals were 20 percent smaller than on untreated animals. Interestingly, DNase 1 treatment appeared to accelerate wound healing in healthy mice, as well.
“The anti-microbial function of NETs may have been more important in the days before antibiotics were common and infections were a more pressing concern for human health,” said Wang. “Now, as humans live longer lives, we may be able to reduce the detrimental effects of NETs in chronic diseases like diabetes, rheumatoid arthritis, and heart disease by controlling expression of the PAD4 enzyme.”
###
In addition to Wang and Wagner, other members of the research team are Siu Ling Wong, Melanie Demers, and Kimberly Martinod from Boston Children's Hospital and Harvard Medical School; Maureen Gallant from Boston Children's Hospital; and Allison B. Goldfine and C. Ronald Kahn from the Joslin Diabetes Center at Harvard Medical School.
The study was supported by the American Diabetes Association (Innovation Award 7-13-IN-44); three institutes of the National Institutes of Health: the National Heart Lung and Blood Institute (grant number R01HL102101), the National Cancer Institute (grant number R01HL136856), and the National Institute of Diabetes and Digestive and Kidney Diseases (grant number R01DK031036); and a GlaxoSmithKline/Immune Disease Institute Alliance Fellowship.
CONTACTS
Yanming Wang: yuw12@psu.edu@psu.edu, 814-441-7404
Barbara Kennedy (PIO): science@psu.edu, 814-863-4682
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

High-energy-density aqueous battery based on halogen multi-electron transfer
Traditional non-aqueous lithium-ion batteries have a high energy density, but their safety is compromised due to the flammable organic electrolytes they utilize. Aqueous batteries use water as the solvent for…

First-ever combined heart pump and pig kidney transplant
…gives new hope to patient with terminal illness. Surgeons at NYU Langone Health performed the first-ever combined mechanical heart pump and gene-edited pig kidney transplant surgery in a 54-year-old woman…

Biophysics: Testing how well biomarkers work
LMU researchers have developed a method to determine how reliably target proteins can be labeled using super-resolution fluorescence microscopy. Modern microscopy techniques make it possible to examine the inner workings…





















