Arylboronates made easy
Using zinc as catalyst, circular molecules can be fitted with two boronate groups at the same time. (Picture: Todd Marder)
Arylboronates are important base materials for the industrial fabrication of countless products, including pharmaceutical drugs, chemicals for agriculture or liquid crystals for displays. The synthesis of arylboronates to date has required metalliferous catalysts such as palladium, iridium or nickel.
These materials have a number of drawbacks: The metals are either expensive, toxic or both. Nickel, for example, can trigger allergies. When used in pharmaceutical drug production, the nickel has to be removed again after the reaction in a complex process.
Progress with zinc catalysts
The Würzburg chemists Shubhankar Kumar Bose and Todd Marder now present an entirely new catalytic process that enables arylboronates to be produced at lower costs and with less environmental impact. Their success is based on the use of zinc catalysts. “Zinc is cheap, non-toxic and abundant on our planet,” Marder names some of the metal's benefits.
As the team reports in “Angewandte Chemie”, a baffling effect occurred during their research work. The scientists haven't been able to fully account for the effect yet, but it should cause a stir among experts, because it might deliver the key to facilitating the synthesis of many important arylboronates in the future.
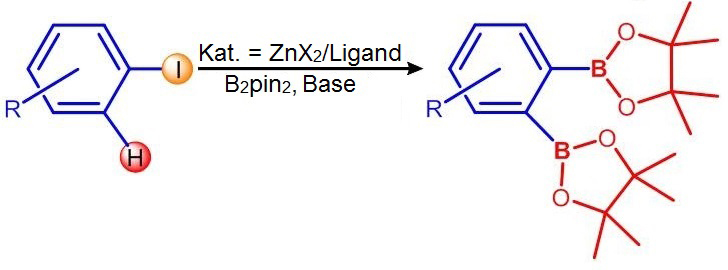
Simultaneous bonding of boronate groups
What caused the astonishment? To produce the arylboronates, circular molecules are used in which either a hydrogen atom or a halogen atom (bromine, fluorine or iodine) is replaced with a so-called boronate group. When the Würzburg scientists initiated this exchange using their zinc catalyst, the two actions happened simultaneously: Both the halogen atom and an adjacent hydrogen atom were replaced by boronate. The result is an aryl with two boronate groups. Usually, these molecules are not so easy to synthesise and they are highly interesting for industrial synthesis.
“This was totally unexpected,” says Marder, “and we don't know yet which chemical mechanism caused the phenomenon.” The chemists already conducted a number of experiments to exclude some reaction channels. Following a process of elimination they hence suggest one possible reaction mechanism in “Angewandte Chemie”.
Next steps in research
In a next step, the scientists want to find out what happened exactly during the reaction with the zinc catalyst. And they are working on increasing the yield of the much sought after substance: The reaction produces around 70 percent of molecules with one boronate group and 30 percent with two boronate groups.
Evolution in catalysis
This success is the preliminary climax of an “evolution of the catalysis” in which Marder's team has played a leading role in the past years. In 1995, the catalysed aryl borylation was successfully conducted in Japan for the first time using palladium; the corresponding reaction is named Miyaura borylation after its inventor.
Arylboronates are needed for the Suzuki-Miyaura reaction. In 2010, Akira Suzuki was awarded the Nobel Prize in Chemistry for its successful implementation. In 2009, Marder's team triggered such reactions using copper catalysts at the University of Durham in England back then. Copper is a low-priced transition metal with low toxicity.
Shubhankar Kumar Bose, who joined the University of Würzburg as a Humboldt scholar in 2013, finally had the idea to try zinc as a catalyst. In 2014, the reaction succeeded first with chain-like molecules (alkylboronates) and now also with circular boronates. Their discovery has another advantage: Zinc is even cheaper than copper and non-toxic.
“Zinc-Catalyzed Dual C–X and C–H Borylation of Aryl Halides“,Shubhankar Kumar Bose, Andrea Deißenberger, Antonius Eichhorn, Patrick G. Steel, Zhenyang Lin, Todd B. Marder. Angewandte Chemie International Edition, published online 18 August 2015, DOI: 10.1002/anie.201505603
Contact
Prof. Dr. Todd Marder, Institute of Inorganic Chemistry, University of Würzburg, Phone +49 931 31-85514, todd.marder@uni-wuerzburg.de
Media Contact
More Information:
http://www.uni-wuerzburg.deAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Making diamonds at ambient pressure
Scientists develop novel liquid metal alloy system to synthesize diamond under moderate conditions. Did you know that 99% of synthetic diamonds are currently produced using high-pressure and high-temperature (HPHT) methods?[2]…

Eruption of mega-magnetic star lights up nearby galaxy
Thanks to ESA satellites, an international team including UNIGE researchers has detected a giant eruption coming from a magnetar, an extremely magnetic neutron star. While ESA’s satellite INTEGRAL was observing…

Solving the riddle of the sphingolipids in coronary artery disease
Weill Cornell Medicine investigators have uncovered a way to unleash in blood vessels the protective effects of a type of fat-related molecule known as a sphingolipid, suggesting a promising new…





















