
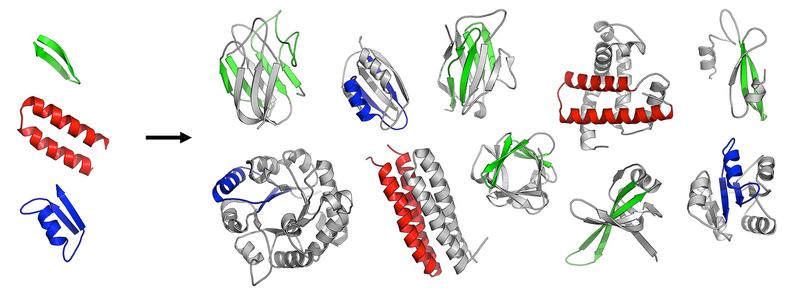
The first folded proteins seem to have originated from an ancestral set of peptides
Vikram Alva/Max Planck Institute for Developmental Biology
In a recent study published in the journal eLife, the scientists report the identification of 40 ancestral, peptidic fragments, which possibly represent the observable remnants of a time when the first proteins were created, more than 3.5 billion years ago.
Proteins are integral building blocks of all life, from bacteria to humans. In our bodies, they are essential for all chemical processes: they form our nails, hair, bones, and muscles, they help digest the food we eat, and they defend us form pathogenic bacteria and viruses.
“Life can be viewed as substantially resulting from the chemical activity of proteins”, says Prof. Andrei Lupas, Director of the Department of Protein Evolution at the Max Planck Institute for Developmental Biology.
He and his collaborators are particularly interested in understanding how these complex biomolecules originated. Today we know that proteins are primarily built through the combinatorial assembly of only a few thousand modular units, termed domains.
It is however unclear how these modular units themselves emerged. Together with Dr. Vikram Alva, first author of the study, and Dr. Johannes Söding, presently head of the Quantitative and Computational Biology Research Group at the Max Planck Institute for Biophysical Chemistry in Göttingen, Prof. Lupas investigated the hypothesis that the first protein domains arose by fusion and piecemeal growth from an ancestral set of simple peptides, which themselves emerged in an RNA-based pre-cellular life, around 3.5 billion years ago.
In a systematic analysis of modern proteins, they were able to identify 40 peptidic fragments that occur in seemingly unrelated proteins, yet bear striking resemblance in their sequences and structures. Based on their widespread occurrence in the most ancient proteins (e.g., ribosomal proteins) and on their involvement in basal functions (e.g., RNA-binding, DNA-binding), the authors propose that these fragments are the observable remnants of a primordial RNA-peptide world, a precursor form of the DNA-based life we know today.
In the future, the contribution of these fragments to the formation of protein structure will have to be investigated experimentally, opening new avenues to optimize existing proteins and design new ones, not yet seen in nature. “If we elucidate this process, we should be able to create new protein forms” concludes Prof. Lupas, with exciting applications to biotechnology.
Original Publication:
A vocabulary of ancient peptides at the origin of folded proteins. Alva V, Söding J, Lupas AN. Elife. 2015 Dec 14;4. pii: e09410. doi: 10.7554/eLife.09410. http://elifesciences.org/content/early/2015/12/14/eLife.
Contact:
Andrei Lupas
Mail: andrei.lupas@tuebingen.mpg.de
Nadja Winter, Press officer (Public Relations)
Phone: +49 7071 601- 444
E-mail: presse-eb@tuebingen.mpg.de
About us:
The Max Planck Institute for Developmental Biology conducts basic research in the fields of biochemistry, genetics and evolutionary biology. It employs about 360 people and is located at the Max Planck Campus in Tübingen. The Max Planck Institute for Developmental Biology is one of 83 research institutes that the Max Planck Society for the Advancement of Science maintains in Germany.
http://elifesciences.org/content/early/2015/12/14/eLife












