
A novel “Kabuto-like” nickel catalyst forms bioactive frameworks from low-cost phenol derivatives
Framework for pharmaceuticals and organic materials
Copyright : ITbM, Nagoya University
<p></p><p></p>
Researchers at ITbM, Nagoya University developed a new nickel catalyst with a “Kabuto-like” structure that was found to catalyze the cross-coupling reaction between carbonyl compounds and readily available phenol derivatives, to form α-arylketones, which are found in many biologically active compounds (Kabuto = a helmet worn by Japanese samurai).
Nagoya, Japan – Professors Kenichiro Itami and Junichiro Yamaguchi of the Institute of Transformative Bio-Molecules (WPI-ITbM) and graduate students Ryosuke Takise and Kei Muto of Nagoya University have succeeded in developing a novel nickel catalyst to catalyze the cross-coupling reaction between carbonyl compounds and phenol derivatives.
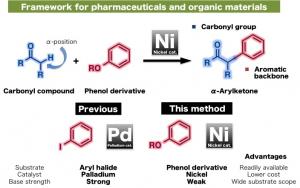
Phenol derivatives are known to be readily available starting materials, and this reaction has enabled the facile generation of α-arylketone compounds (aryl = aromatic ring; ketone = a carbonyl (C=O functionality with a carbon oxygen double bond) bonded to two other carbon atoms), which constitute the framework of many pharmaceuticals and organic materials.
The study published online on May 20, 2014 in Angewandte Chemie International Edition, is expected to become an important tool in synthesizing biologically active molecules and a range of versatile organic materials containing the α-arylketone framework.
Cross-coupling reactions catalyzed by transition metals enables direct formation of carbon-carbon bonds, which is widely utilized in the formation of a vast number of organic frameworks. This strategy that won the 2010 Nobel Prize in Chemistry extends to α-arylation reactions, which allows incorporation of aromatic rings into carbonyl compounds through carbon-carbon bond formation, mainly using palladium as a catalyst. Many organic molecules such as amino acids contain carbonyl groups.
Thus, α-arylation, which links carbonyl compounds to aromatic rings present in an array of organic molecules, has been used as one of the main reactions to create the organic backbone of many pharmaceuticals and organic materials. Professors Itami and Yamaguchi along with co-workers have been working to develop a new economical catalyst to conduct α-arylation reactions using readily available phenol derivatives as starting materials. This reaction system is envisaged to lead to the facile production of α-arylketones on an industrial scale.
Conventional methods for α-arylation have used palladium as a metal catalyst. “Since 2009, we have been looking at the potential of nickel as a cross-coupling catalyst, which is a more economical catalyst relative to palladium”, says Professor Yamaguchi, one of the leaders of this research, “through our endeavors to develop improved conditions, we have found that phenol derivatives can act as readily available aryl coupling partners”, he describes. Aryl substrates have mainly utilized aryl halides, which are halogenated compounds resulting in toxic wastes. Phenol derivatives on the other hand are usually less toxic and are more readily available compared to aryl halides.
Itami and his group have been working on the development of new generation cross-coupling methods using nickel as a catalyst. They have already reported various cross-coupling reactions including those between aromatic compounds and phenol derivatives, C-H coupling reactions using esters along with reactions linking aromatic rings and alkenes. These pioneering studies have been accomplished by enhancing the reactivity of nickel through tuning of various ligands. One of the effective ligands discovered in 2011 is the dcype (1,2-bisdicyclohexylphosphinoethane) ligand, which is used with nickel to activate phenol derivatives. However, the nickel(dcype) catalyst was insufficient to initiate the cross-coupling between carbonyl compounds and phenol derivatives.
“We have screened various ligands and found that dcypt (1,2-bisdicyclohexylphosphinothiophene), which bears a thiophene (a 5-membered heterocycle containing sulfur) unit, acts as an effective air-stable ligand to improve the reactivity of nickel and bring about this reaction in high yield. Moreover, we were able to use a weak base to carry out this reaction, which improves the substrate scope of this reaction”, explains graduate students Takise and Muto who carried out the experiments.
Development of this new nickel catalyst has enabled the creation of a variety of α-arylketone compounds, including an estrone derivative and an amino acid tyrosine derivative, both bearing the phenol moiety. Professor Itami elaborates, “Many natural products and bioactive compounds contain the α-arylketone framework. Our relatively low-cost nickel-dcypt catalyst has exhibited high activity to couple readily available phenol derivatives in high yield. This system has the potential to be applicable in the synthesis of molecules that can control biological systems of plants and animals, which is one of the main research themes running in our new institute (ITbM).”
This article “Nickel-Catalyzed α-Arylation of Ketones with Phenol Derivatives” by Ryosuke Takise, Kei Muto, Junichiro Yamaguchi and Kenichiro Itami is published online on May 20, 2014 in Angewandte Chemie International Edition.
DOI: 10.1002/anie.201403823
About WPI-ITbM (http://www.itbm.nagoya-u.ac.jp/)
The World Premier International Research Center Initiative (WPI) for the Institute of Transformative Bio-Molecules (ITbM) at Nagoya University in Japan is committed to advance the integration of synthetic chemistry, plant/animal biology and theoretical science, all of which are traditionally strong fields in the university. As part of the Japanese science ministry’s MEXT program, the ITbM aims to develop transformative bio-molecules, innovative functional molecules capable of bringing about fundamental change to biological science and technology. Research at the ITbM is carried out in a “Mix-Lab” style, where international young researchers from multidisciplinary fields work together side-by-side in the same lab. Through these endeavors, the ITbM will create “transformative bio-molecules” that will dramatically change the way of research in chemistry, biology and other related fields to solve urgent problems, such as environmental issues, food production and medical technology that have a significant impact on the society.
Author Contact
Professor Kenichiro Itami
Institute of Transformative Bio-Molecules (WPI-ITbM), Nagoya University
Furo-Cho, Chikusa-ku, Nagoya 464-8601, Japan
TEL/FAX: +81-52-788-6098
E-mail: itami@chem.nagoya-u.ac.jp
URL for Group Homepage: http://synth.chem.nagoya-u.ac.jp/
URL for ITbM Homepage: http://www.itbm.nagoya-u.ac.jp/
Public Relations Contact
Dr. Ayako Miyazaki
Institute of Transformative Bio-Molecules (WPI-ITbM), Nagoya University
Furo-Cho, Chikusa-ku, Nagoya 464-8601, Japan
TEL: +81-52-789-4999 FAX: +81-52-789-3240
E-mail: press@itbm.nagoya-u.ac.jp
Nagoya University Public Relations Office
TEL: +81-52-789-2016 FAX: +81-52-788-6272
E-mail: kouho@adm.nagoya-u.ac.jp












