One-pot synthesis towards sulfur-based organic semiconductors
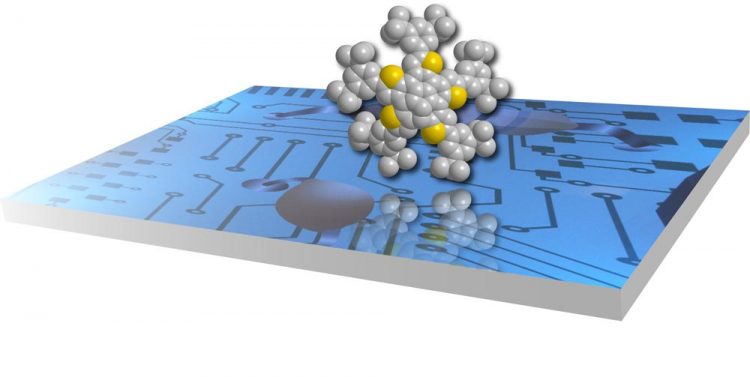
Yellow and gray colors on the molecule represent sulfur and carbon atoms respectively. Thiophene-fused PAHs have found uses as transistors. Copyright : ITbM, Nagoya University
Thiophene-fused polycyclic aromatic hydrocarbons (PAHs) are known to be useful as organic semiconductors due to their high charge transport properties. Scientists at Nagoya University have developed a short route to form various thiophene-fused PAHs by simply heating mono-functionalized PAHs with sulfur. This new method is expected to contribute towards the efficient development of novel thiophene-based electronic materials.
Nagoya, Japan – Dr. Lingkui Meng, Dr. Yasutomo Segawa, Professor Kenichiro Itami of the JST-ERATO Itami Molecular Nanocarbon Project, Institute of Transformative Bio-Molecules (ITbM) of Nagoya University and Integrated Research Consortium on Chemical Sciences, and their colleagues have reported in the Journal of the American Chemical Society, on the development of a simple and effective method for the synthesis of thiophene-fused PAHs.
Thiophene-fused PAHs are organic molecules composed of multiple aromatic rings including thiophene. Thiophene is a five-membered aromatic ring containing four carbon atoms and a sulfur atom. Thiophene-fused PAHs are known to be one of the most common organic semiconductors and are used in various electronic materials, such as in transistors, organic thin-film solar cells, organic electro-luminescent diodes and electronic devices. More recently, they have found use in wearable devices due to their lightweight and flexibility.
Thienannulation (thiophene-annulation) reactions, a transformation that makes new thiophene rings via cyclization, leads to various thiophene-fused PAHs. Most conventional thienannulation methods require the introduction of two functional groups adjacent to each other to form two reactive sites on PAHs before the cyclization can take place. Thus, multiple steps are required for the preparation of the substrates. As a consequence, a more simple method to access thiophene-fused PAHs is desirable.
A team led by Yasutomo Segawa, a group leader of the JST-ERATO project, and Kenichiro Itami, the director of the JST-ERATO project and the center director of ITbM, has succeeded in developing a simple and effective method for the formation of various thiophene-fused PAHs. They have managed to start from PAHs that have only one functional group, which saves the effort of installing another functional group, and have performed the thienannulation reactions using elemental sulfur, a readily available low cost reagent. The reactions can be carried out on a multigram scale and can be conducted in a one-pot two-step reaction sequence starting from an unfunctionalized PAH. This new approach can also generate multiple thiophene moieties in a single reaction. Hence, this method has the advantage of offering a significant reduction in the number of required steps and in the reagent costs for thiophene-fused PAH synthesis compared to conventional methods.
The researchers have shown that upon heating and stirring the dimethylformamide solution of arylethynyl group-substituted PAHs and elemental sulfur in air, they were able to obtain the corresponding thiophene-fused PAHs. The arylethynyl group consists of an alkyne (a moiety with a carbon-carbon triple bond) bonded to an aromatic ring. The reaction proceeds via a carbon-hydrogen (C–H) bond cleavage at the position next to the arylethynyl group (called the ortho-position) on PAHs, in the presence of sulfur. As the ortho-C–H bond on the PAH can be cleaved under the reaction conditions, prior functionalization (installation of a functional group) becomes unnecessary.
Arylethynyl-substituted PAHs are readily accessible by the Sonogashira coupling, which is a cross-coupling reaction to form carbon-carbon bonds between an alkyne and a halogen-substituted aromatic compound. The synthesis of thiophene-fused PAHs can also be carried out in one-pot, in which PAHs are subjected to a Sonogashira coupling to form arylethynyl-substituted PAHs, followed by direct treatment of the alkyne with elemental sulfur to induce thienannulation.
“Actually, we coincidentally discovered this reaction when we were testing different chemical reactions to synthesize a new molecule for the Itami ERATO project,” says Yasutomo Segawa, one of the leaders of this study. “At first, most members including myself felt that the reaction may have already been reported because it is indeed a very simple reaction. Therefore, the most difficult part of this research was to clarify the novelty of this reaction. We put in a significant amount of effort to investigate previous reports, including textbooks from more than 50 years ago as well as various Internet sources, to make sure that our reaction conditions had not been disclosed before,” he continues.
The team succeeded in synthesizing more than 20 thiophene-fused PAHs. They also revealed that multiple formations of thiophene rings of PAHs substituted with multiple arylethynyl groups could be carried out all at once. Multiple thiophene-fused PAHs were generated from three-fold and five-fold thienannulations, which generated triple thia[5]helicene (containing three thiophenes) and pentathienocorannulene (containing five thiophenes), respectively. The pentathienocorannulene was an unprecedented molecule that was synthesized for the first time by the group's new method.
“I was extremely happy when I was able to obtain the propeller-shaped triple thia[5]helicene and hat-shaped pentathienocorannulene, because I have always been aiming to synthesize exciting new molecules since I joined Professor Itami’s group,” says Lingkui Meng, a postdoctoral researcher who mainly conducted the experiments. “We had some problems in purifying the compounds but we were delighted when we obtained the crystal structures of the thiophene compounds, which proved that the desired reactions had taken place.”
“The best part of this research for me is to discover that our C-H functionalization strategy on PAHs could be applied to synthesize structurally beautiful molecules with high functionalities,” says Segawa. “The successful synthesis of a known high-performance organic semiconductive molecule, (2,6-bis(4-n-octylphenyl)- dithieno[3,2-b:2′,3′-d]thiophene, from a relatively cheap substrate opens doors to access useful thiophene compounds in a rapid and cost-effective manner.”
“We hope that ongoing advances in our method may lead to the development of new organic electronic devices, including semiconductor and luminescent materials,” say Segawa and Itami. “We are considering the possibilities to make this reaction applicable for making useful thiophene-fused PAHs, which would lead to the rapid discovery and optimization of key molecules that would advance the field of materials science.”
JST-ERATO Itami Molecular Nanocarbon Project
JST-ERATO Itami Molecular Nanocarbon Project was launched at Nagoya University in April 2014. This is a 5-year project that seeks to open the new field of nanocarbon science. This project entails the design and synthesis of as-yet largely unexplored nanocarbons as structurally well-defined molecules, and the development of novel, highly functional materials based on these nanocarbons. Researchers combine chemical and physical methods to achieve the controlled synthesis of well-defined uniquely structured nanocarbon materials, and conduct interdisciplinary research encompassing the control of molecular arrangement and orientation, structural and functional analysis, and applications in devices and biology. The goal of this project is to design, synthesize, utilize, and understand nanocarbons as molecules.
About WPI-ITbM
The Institute of Transformative Bio-Molecules (ITbM) at Nagoya University in Japan is committed to advance the integration of synthetic chemistry, plant/animal biology and theoretical science, all of which are traditionally strong fields in the university. ITbM is one of the research centers of the Japanese MEXT (Ministry of Education, Culture, Sports, Science and Technology) program, the World Premier International Research Center Initiative (WPI). The aim of ITbM is to develop transformative bio-molecules, innovative functional molecules capable of bringing about fundamental change to biological science and technology. Research at ITbM is carried out in a “Mix-Lab” style, where international young researchers from various fields work together side-by-side in the same lab, enabling interdisciplinary interaction. Through these endeavors, ITbM will create “transformative bio-molecules” that will dramatically change the way of research in chemistry, biology and other related fields to solve urgent problems, such as environmental issues, food production and medical technology that have a significant impact on the society.
About JST-ERATO
ERATO (The Exploratory Research for Advanced Technology), one of the Strategic Basic Research Program, aims to form a headstream of science and technology, and ultimately contribute to science, technology, and innovation that will change society and the economy in the future. In ERATO, a Research Director, a principal investigator of ERATO research project, establishes a new research base in Japan and recruits young researchers to implement his or her challenging research project within a limited time frame.
Journal information This article “Thiophene-Fused π-Systems from Diarylacetylenes and Elemental Sulfur” by Lingkui Meng, Takao Fujikawa, Motonobu Kuwayama, Yasutomo Segawa and Kenichiro Itami is published online in the Journal of the American Chemical Society. DOI: 10.1021/jacs.6b06486 (http://dx.doi.org/10.1021/jacs.6b06486)
Media Contact
More Information:
http://www.researchsea.comAll latest news from the category: Power and Electrical Engineering
This topic covers issues related to energy generation, conversion, transportation and consumption and how the industry is addressing the challenge of energy efficiency in general.
innovations-report provides in-depth and informative reports and articles on subjects ranging from wind energy, fuel cell technology, solar energy, geothermal energy, petroleum, gas, nuclear engineering, alternative energy and energy efficiency to fusion, hydrogen and superconductor technologies.
Newest articles

Silicon Carbide Innovation Alliance to drive industrial-scale semiconductor work
Known for its ability to withstand extreme environments and high voltages, silicon carbide (SiC) is a semiconducting material made up of silicon and carbon atoms arranged into crystals that is…

New SPECT/CT technique shows impressive biomarker identification
…offers increased access for prostate cancer patients. A novel SPECT/CT acquisition method can accurately detect radiopharmaceutical biodistribution in a convenient manner for prostate cancer patients, opening the door for more…

How 3D printers can give robots a soft touch
Soft skin coverings and touch sensors have emerged as a promising feature for robots that are both safer and more intuitive for human interaction, but they are expensive and difficult…