
Superoxide Boosts Lithium-Air Batteries: DOE’s Latest Breakthrough
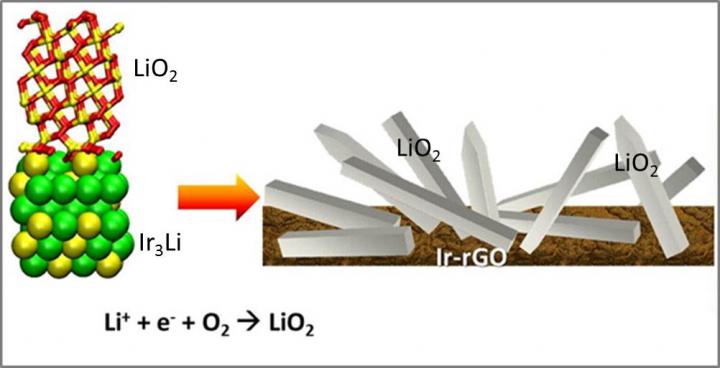
The lattice match between LiO2 and Ir3Li may be responsible for the LiO2 discharge product found for the Ir-rGO cathode material.
Credit: Argonne/Larry Curtiss
Now, thanks to research at the U.S. Department of Energy's (DOE) Argonne National Laboratory, one of those drawbacks may have been overcome.
All previous work on lithium-air batteries showed the same phenomenon: the formation of lithium peroxide (Li2O2), a solid precipitate that clogged the pores of the electrode.
In a recent experiment, however, Argonne battery scientists Jun Lu, Larry Curtiss and Khalil Amine, along with American and Korean collaborators, were able to produce stable crystallized lithium superoxide ((LiO2) instead of lithium peroxide during battery discharging. Unlike lithium peroxide, lithium superoxide can easily dissociate into lithium and oxygen, leading to high efficiency and good cycle life.
“This discovery really opens a pathway for the potential development of a new kind of battery,” Curtiss said. “Although a lot more research is needed, the cycle life of the battery is what we were looking for.”
The major advantage of a battery based on lithium superoxide, Curtiss and Amine explained, is that it allows, at least in theory, for the creation of a lithium-air battery that consists of what chemists call a “closed system.” Open systems require the consistent intake of extra oxygen from the environment, while closed systems do not – making them safer and more efficient.
“The stabilization of the superoxide phase could lead to developing a new closed battery system based on lithium superoxide, which has the potential of offering truly five times the energy density of lithium ion,” Amine said.
Curtiss and Lu attributed the growth of the lithium superoxide to the spacing of iridium atoms in the electrode used in the experiment. “It looks like iridium will serve as a good template for the growth of superoxide,” Curtiss said.
“However, this is just an intermediate step,” Lu added. “We have to learn how to design catalysts to understand exactly what's involved in lithium-air batteries.”
###
The researchers confirmed the lack of lithium peroxide by using X-ray diffraction provided by the Advanced Photon Source, a DOE Office of Science User Facility located at Argonne. They also received allocations of time on the Mira supercomputer at the Argonne Leadership Computing Facility, which is also a DOE Office of Science User Facility. The researchers also performed some of the work at Argonne's Center for Nanoscale Materials, which is also a DOE Office of Science User Facility.
A study based on the research appeared in the January 11 issue of Nature.
The work was funded by the DOE's Office of Energy Efficiency and Renewable Energy and Office of Science.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit the Office of Science website.
For more information, please contact Jared Sagoff at jsagoff@anl.gov or 630-252-5549.












