
Capture Carbon Dioxide and Transform It Into Valuable Products
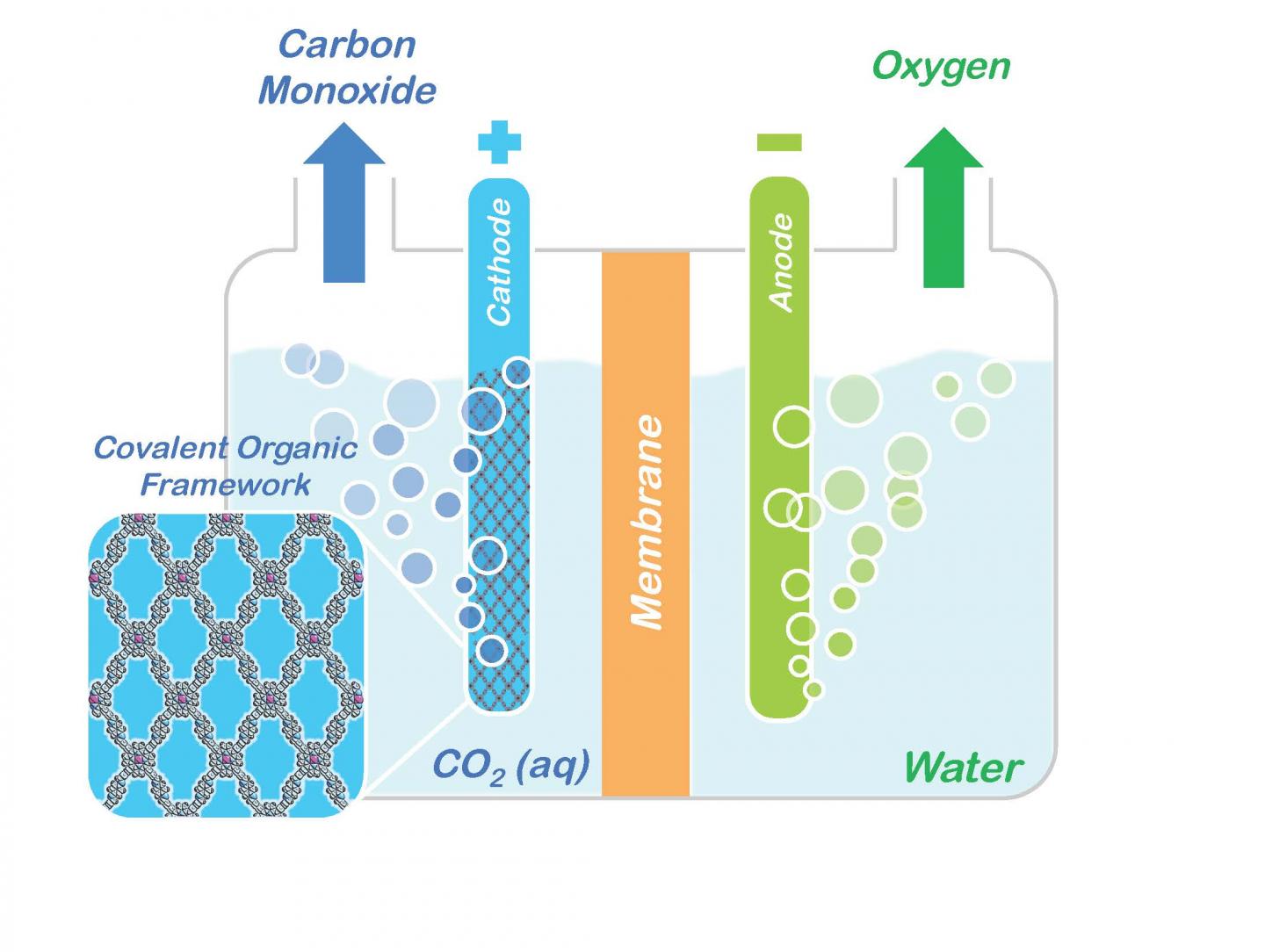
Conceptual model shows how porphyrin COFs embedded in a cathode could be used to split carbon dioxide (CO2) into carbon monoxide (CO) and oxygen for making renewable fuels and other valuable chemical products.
Courtesy of Omar Yaghi, Berkeley Lab/UC Berkeley
A molecular system that holds great promise for the capture and storage of carbon dioxide has been modified so that it now also holds great promise as a catalyst for converting captured carbon dioxide into valuable chemical products. Researchers with the U.S. Department of Energy (DOE)'s Lawrence Berkeley National Laboratory (Berkeley Lab) have incorporated molecules of carbon dioxide reduction catalysts into the sponge-like crystals of covalent organic frameworks (COFs). This creates a molecular system that not only absorbs carbon dioxide, but also selectively reduces it to carbon monoxide, which serves as a primary building block for a wide range of chemical products including fuels, pharmaceuticals and plastics.
“There have been many attempts to develop homogeneous or heterogeneous catalysts for carbon dioxide, but the beauty of using COFs is that we can mix-and-match the best of both worlds, meaning we have molecular control by choice of catalysts plus the robust crystalline nature of the COF,” says Christopher Chang, a chemist with Berkeley Lab's Chemical Sciences Division, and a co-leader of this study. “To date, such porous materials have mainly been used for carbon capture and separation, but in showing they can also be used for carbon dioxide catalysis, our results open up a huge range of potential applications in catalysis and energy.”
Chang and Omar Yaghi, a chemist with Berkeley Lab's Materials Sciences Division who invented COFs, are the corresponding authors of a paper in Science that describes this research in detail. The paper is titled “Covalent organic frameworks comprising cobalt porphyrins for catalytic CO2 reduction in water.” Lead authors are Song Lin, Christian Diercks and Yue-Biao Zhang. Other co-authors are Nikolay Kornienko, Eva Nichols, Yingbo Zhao, Aubrey Paris, Dohyung Kim and Peidong Yang.
Chang and Yaghi both hold appointments with the University of California (UC) Berkeley. Chang is also a Howard Hughes Medical Institute (HHMI) investigator. Yaghi is co-director of the Kavli Energy NanoScience Institute (Kavli-ENSI) at UC Berkeley.
The notoriety of carbon dioxide for its impact on the atmosphere and global climate change has overshadowed its value as an abundant, renewable, nontoxic and nonflammable source of carbon for the manufacturing of widely used chemical products. With the reduction of atmospheric carbon dioxide emissions in mind, Yaghi and his research group at the University of Michigan in 2005 designed and developed the first COFs as a means of separating carbon dioxide from flue gases. A COF is a porous three-dimensional crystal consisting of a tightly folded, compact framework that features an extraordinarily large internal surface area – a COF the size of a sugar cube were it to be opened and unfolded would blanket a football field. The sponge-like quality of a COF's vast internal surface area enables the system to absorb and store enormous quantities of targeted molecules, such as carbon dioxide.
Now, through another technique developed by Yaghi, called “reticular chemistry,” which enables molecular systems to be “stitched” into netlike structures that are held together by strong chemical bonds, the Berkeley Lab researchers were able to embed the molecular backbone of COFs with a porphyrin catalyst, a ring-shaped organic molecule with a cobalt atom at its core. Porphyrins are electrical conductors that are especially proficient at transporting electrons to carbon dioxide.
“A key feature of COFs is the ability to modify chemically active sites at will with molecular-level control by tuning the building blocks constituting a COF's framework,” Yaghi says. “This affords a significant advantage over other solid-state catalysts where tuning the catalytic properties with that level of rational design remains a major challenge. Because the porphyrin COFs are stable in water, they can operate in aqueous electrolyte with high selectivity over competing water reduction reactions, an essential requirement for working with flue gas emissions.”
In performance tests, the porphyrin COFs displayed exceptionally high catalytic activity – a turnover number up to 290,000, meaning one porphyrin COF can reduce 290,000 molecules of carbon dioxide to carbon monoxide every second. This represents a 60-fold increase over the catalytic activity of molecular cobalt porphyrin catalyst and places porphyrin COFs among the fastest and most efficient catalysts of all known carbon dioxide reduction agents. Furthermore, the research team believes there's plenty of room for further improving porphyrin COF performances.
“We're now seeking to increase the number of electroactive cobalt centers and achieve lower over-potentials while maintaining high activity and selectivity for carbon dioxide reduction over proton reduction,” Chang says. “In addition we are working towards expanding the types of value-added carbon products that can be made using COFs and related frameworks.”
###
This research was supported by the DOE Office of Science in part through its Energy Frontier Research Center (EFRC) program. The porphyrin COFs were characterized through X-ray absorption measurements performed at Berkeley Lab's Advanced Light Source, a DOE Office of Science User Facility.
Lawrence Berkeley National Laboratory addresses the world's most urgent scientific challenges by advancing sustainable energy, protecting human health, creating new materials, and revealing the origin and fate of the universe. Founded in 1931, Berkeley Lab's scientific expertise has been recognized with 13 Nobel prizes. The University of California manages Berkeley Lab for the U.S. Department of Energy's Office of Science. For more, visit http://www.
DOE's Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit the Office of Science website at science.energy.gov/.









![[1] Key Research Findings on Transfer-Printed Alumina–Gold Dual-layer Protective Coating](https://www.innovations-report.com/wp-content/uploads/2025/07/Transfer_printing_technology_for_lithium_protectiv_1753252750-e1753252940671-362x245.jpg)


