
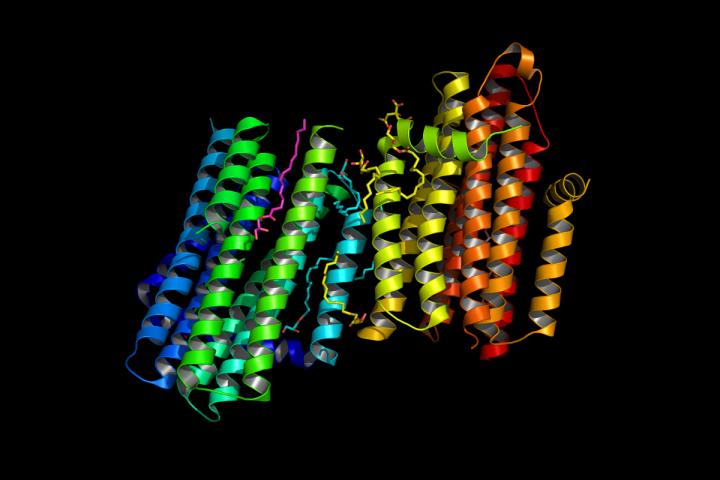
Graphic shows the structure of diacylglycerol kinase (DgkA) determined with the Free Electron Laser (FEL) in Stanford, Ca. This new structure has been deposited in the protein data bank under the code 4UYO.
Credit: The Biodesign Institute at Arizona State University
The scientists produced the structural map of this nanomachine – diacylglycerol kinase – by using a “hit and run” crystallography technique. In doing so, they have been able to understand how the tiny enzyme performs critical cellular duties – answering questions that have been on the table for over 50 years about this 'paradigmatic protein'.
Kinases are key players in metabolism, cell signalling, protein regulation, cellular transport, secretory processes, and many other cellular pathways that allow us to function healthily. They coordinate the transfer of energy from certain molecules to specific substrates, affecting their activity, reactivity, and ability to bind other molecules.
Diacylglycerol kinase, the focus of this study, plays a role in bacterial cell wall synthesis. It is a small, integral membrane enzyme that coordinates a particularly complex reaction: its lipid substrate is hydrophobic (repelled by water) and resides in cell membranes, while its co-substrate, ATP, is entirely water soluble.
How it does this had remained a mystery for decades, but the newly produced blueprints have answered these questions.
“How this diminutive nanomachine, less than 10 nm tall, brings these two disparate substrates together at the membrane interface for reaction is revealed in a molecularly detailed crystal structure. It is the smallest known kinase, and seeing its form with crystal clarity is now helping us to answer questions that formed from over 50 years of work on this paradigmatic protein,” said Professor of Membrane Structural and Functional Biology at Trinity College Dublin, Martin Caffrey.
Figuring out how this tiny machine works at the molecular level was enormously facilitated by our use of one of the brightest X-ray sources on Earth, the X-ray free-electron laser at the Stanford Linear Accelerator Center.
Professor Caffrey added: “This instrument produces bursts of X-rays just femtoseconds (a quad-trillionth of a second) long. With these short bursts we were able to obtain structural information about the enzyme before it vaporized through radiation damage in what I tritely refer to as 'Hit and Run' serial crystallography.”
According to Petra Fromme, the director of the Center for Applied Structural Discovery at Arizona State University's Biodesign Institute and a co-author of the current study, “this is the first structure of a protein that is a membrane-integral enzyme and important biocatalyst in the cell.” (Biocatalysts speed up the rate of critical biological reactions.)
The tiny kinase is one of the research targets for the NIH funded Center for Membrane Proteins in Infectious Diseases at ASU, which is devoted to unraveling the molecular basis of viral and bacterial proteins involved in diseases as well as the human proteins defending the body from pathogen attack.
The ASU team contributed to the work with expertise in crystal growth and sample injection, as well as data collection and evaluation.
In the future, the scientists hope to extend their free-electron laser work to make 'X-ray movies' of this remarkable nanomachine, so as to watch how it 'does chemistry' in atomic detail in real time.
The article describing the work has just been published in the leading journal Nature Communications.
###
The team of researchers at ASU includes the faculty Wei Liu, Petra Fromme and Raimund Fromme from the School of Molecular Sciences, and John Spence, Uwe Weierstall and Nadia Zatsepin from the Department of Physics, the researcher Ingo Grotjohann as well as the graduate students Shibom Basu, Christopher Kupitz and Kimberley Rendek.
Media Contact
Petra Fromme, Professor and Director, Center for Applied Structural Discovery, Biodesign Institute at Arizona State University. Petra Fromme Petra.Fromme@asu.edu or tel: 480)965-9028 Lab Phone: (480)965-8040
Richard Harth, Science Writer, Biodesign Institute at Arizona State University. Richard.harth@asu.edu
Martin Caffrey, Professor of Membrane Structural and Functional Biology, School of Biochemistry and Immunology, Trinity College Dublin, at martin.caffrey@tcd.ie or Tel: +353-1-896-4253 / +353-86-818-7704
Thomas Deane, Press Officer for the Faculty of Engineering, Mathematics and Science, Trinity College Dublin, at deaneth@tcd.ie or Tel: +353-1-896-4685












