Research reveals mechanism for direct synthesis of hydrogen peroxide
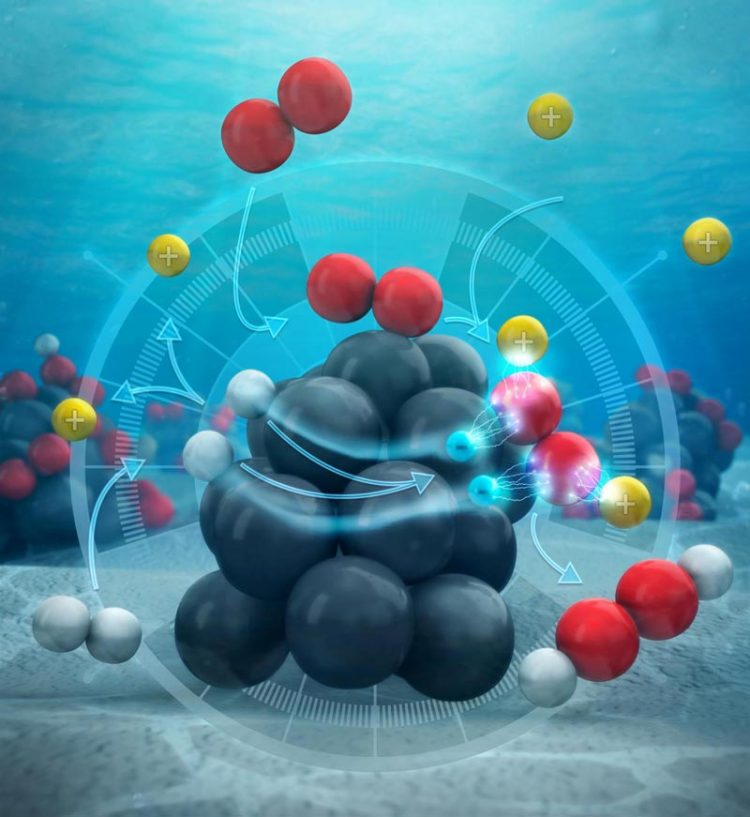
Instead of reacting together on the surface of the catalyst (the palladium cluster), the hydrogen atoms dissociate into their components -- protons and electrons. The protons enter the surrounding solution of water and methanol, while the electrons flow through the palladium itself into oxygen molecules. Credit: American Chemical Society
As a result, scientists and companies have been exploring a more environmentally benign alternative to chlorine–hydrogen peroxide, or H2O2. But it is an expensive reactant. Hydrogen peroxide is typically made in big, centralized facilities and requires significant energy for separation, concentration, and transportation.
A handful of large-scale facilities around the globe have begun to produce H2O2 using the current process, but at the same facilities as the polyurethane precursors, which results in significant cost and energy savings and reduces environmental impact.
Ideally smaller-scale factories would also be able to make hydrogen peroxide on site, but this would require a completely different set of chemistry, direct synthesis of H2O2 from hydrogen and oxygen gas, which has long been poorly understood according to researchers at the University of Illinois at Urbana-Champaign.
New research from David Flaherty, assistant professor of chemical and biomolecular engineering, and graduate student Neil Wilson reveals the mechanism for the direct synthesis of H2O2 on palladium cluster catalysts, and paves the way to design improved catalysts to produce H2O2 to use in place of harmful chlorine, regardless of the scale of the production facility. The research appears as the cover story for the Jan. 20, 2016 issue of the Journal of the American Chemical Society.
The commonly accepted mechanism for direct synthesis of H2O2 essentially states that hydrogen and oxygen atoms bind adjacent to one another on the catalyst surface and then react, Wilson said. To better understand what was going on, he spent over a year building a reactor, fine-tuning experimental procedures, then collecting and analyzing reaction rate data.
“What people thought was happening is after the hydrogen atoms broke apart and they're adsorbed onto the palladium surface, that they just reacted with the oxygen on the surface. But that's not really consistent with what we saw,” said Wilson, a fourth-year graduate student in Flaherty's lab and first author of the article, “Mechanism for the Direct Synthesis of H2O2 on Pd Clusters: Heterolytic Reaction Pathways at the Liquid-Solid Interface.”
Featured on the journal's cover is an image that depicts their findings: Instead of reacting together on the surface of the catalyst (the palladium cluster), the hydrogen atoms dissociate into their components–protons and electrons. The protons enter the surrounding solution of water and methanol, while the electrons flow through the palladium itself into oxygen molecules.
“When oxygen comes down onto the surface, it can react with pairs of protons and electrons to form hydrogen peroxide,” Wilson said.
“The reason this is critical,” Flaherty said, “is because it gives us guidance for how to make the next generation of these materials. This is all motivated by trying to make hydrogen peroxide more cheaply so it can be manufactured more easily, so we can use it in place of chlorine. But we didn't know how to go about making a catalyst that was better than what we have now.”
Researchers will now have a better sense of what is happening at the catalyst surface and an appreciation for the role of proton and electron transfer processes in this chemistry. It was not recognized that the oxygen on the surface reacted with liquid phase species, and that the formation of H2O2 by direct synthesis is, therefore, strongly influenced by the solution itself. However, the formation of water (the undesired side reaction) is mostly influenced by properties of the catalyst surface.
“Now that we understand what's happening on the surface, we can start pushing towards rational catalyst design,” Wilson said. The research group is now looking into another catalyst, gold-palladium, which has been shown in previous work to be very selective towards H2O2. “People still don't entirely know why gold-palladium is so selective,” Wilson added, but it seems that this new mechanistic insight will help to explain the selectivity of these materials.
Several students in Flaherty's lab are currently exploring different ways of “coupling this chemistry directly with reactions that use hydrogen peroxide for green oxidations within very short length scales,” that is, micrometers away, Flaherty said. “If we can put these H2O2 formation catalysts very close to something which performs the oxidation reaction, we can avoid the entire problem or concentrating and transporting hydrogen peroxide.”
Media Contact
All latest news from the category: Materials Sciences
Materials management deals with the research, development, manufacturing and processing of raw and industrial materials. Key aspects here are biological and medical issues, which play an increasingly important role in this field.
innovations-report offers in-depth articles related to the development and application of materials and the structure and properties of new materials.
Newest articles

High-energy-density aqueous battery based on halogen multi-electron transfer
Traditional non-aqueous lithium-ion batteries have a high energy density, but their safety is compromised due to the flammable organic electrolytes they utilize. Aqueous batteries use water as the solvent for…

First-ever combined heart pump and pig kidney transplant
…gives new hope to patient with terminal illness. Surgeons at NYU Langone Health performed the first-ever combined mechanical heart pump and gene-edited pig kidney transplant surgery in a 54-year-old woman…

Biophysics: Testing how well biomarkers work
LMU researchers have developed a method to determine how reliably target proteins can be labeled using super-resolution fluorescence microscopy. Modern microscopy techniques make it possible to examine the inner workings…





















